Scoping review: (Bio)markers for the prognostication of breast cancer recurrence
- PMID: 40597870
- PMCID: PMC12211913
- DOI: 10.1186/s12885-025-14515-z
Scoping review: (Bio)markers for the prognostication of breast cancer recurrence
Abstract
Background: The aim of this study is to gain a comprehensive understanding of the latest advancements in breast cancer recurrence markers, with the aim of identifying minimally invasive or minimally intrusive markers as necessary approach for screening for breast cancer recurrence.
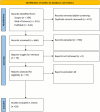
Methods: We followed PRISMA guidelines, systematically searching Web of Science, Scopus, and PubMed from 2010 to December 2023 for secondary papers on breast cancer markers of recurrence. Keywords used to search the databases include but are not limited to: "breast cancer recurrence", "markers", "radiology", "pathology", "clinical features". Studies focusing solely on outcomes after recurrence, such as survival or treatment response, were excluded to ensure the review targeted markers relevant to early prediction. The search was limited to English language. Selected papers underwent screening process according to inclusion/exclusion criteria, and data extraction included publication details, markers, marker modality, among others.
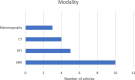
Results: The number of papers considered for this review was 1,138. After two phases of screening process, a total number of 28 reviews were included in this scoping review. We have categorized markers into radiological, clinical, and histopathological types. Among the most relevant clinical markers correlated with breast cancer (BC) recurrence are clinical stage, carcinoembryogenic antigen (CEA), and cancer antigen 15.3 (CA 15.3). We have also identified that the following radiological markers are the most mentioned markers associated with recurrence: mammographic density (MD), tumor heterogeneity, most enhancing tumor volume (METV), radiomic features, and more. Furthermore, we identified nuclear grade, microenvironment heterogeneity, estrogen receptor (ER), androgen receptor (AR), human epidermal growth factor receptor 2 (HER2), Ki-67 antigen, as the most significant histopathological markers of breast cancer recurrence.
Conclusion: This review identified promising markers for breast cancer recurrence in three categories: clinical, radiological and histopathological. General practitioners can leverage these insights for enhanced pre-screening, aiding in earlier detection and intervention, thus improving patient outcomes. Unclear cut-off values and disagreement on their use remain obstacles.
Keywords: Breast cancer recurrence; Clinical markers; Histopathological markers; Minimally invasive markers; Prognostic; Radiological markers; Scoping review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The research was carried out under PRISMA methodology, no specific ethics approval was required. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Blood biomarkers for the non-invasive diagnosis of endometriosis.Cochrane Database Syst Rev. 2016 May 1;2016(5):CD012179. doi: 10.1002/14651858.CD012179. Cochrane Database Syst Rev. 2016. PMID: 27132058 Free PMC article.
-
Mammographic density, endocrine therapy and breast cancer risk: a prognostic and predictive biomarker review.Cochrane Database Syst Rev. 2021 Oct 26;10(10):CD013091. doi: 10.1002/14651858.CD013091.pub2. Cochrane Database Syst Rev. 2021. PMID: 34697802 Free PMC article.
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. 10.3322/caac.21708. - PubMed
-
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–73. 10.7326/M18-0850. - PubMed
-
- Evangelista L, Cervino AR, Ghiotto C, Al-Nahhas A, Rubello D, Muzzio PC. Tumor marker-guided PET in breast cancer patients—a recipe for a perfect wedding: a systematic literature review and meta-analysis. Clin Nucl Med. 2012;37:467. 10.1097/RLU.0b013e31824850b0. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous