Sulforaphane induces cell morphology change and cell apoptosis by activating endoplasmic reticulum stress in glioblastoma
- PMID: 40597933
- PMCID: PMC12210932
- DOI: 10.1186/s12885-025-14378-4
Sulforaphane induces cell morphology change and cell apoptosis by activating endoplasmic reticulum stress in glioblastoma
Abstract
Background: Sulforaphane (SFN), a naturally occurring isothiocyanate derived from cruciferous vegetables, has shown promise as a multitargeted therapeutic agent in glioblastoma (GBM). This study aimed to elucidate the role and underlying molecular mechanisms of SFN in regulating GBM progression, particularly through the endoplasmic reticulum stress (ERS) and unfolded protein response (UPR) pathways.
Methods: Primary human glioma cells and established GBM cell lines were treated with various concentrations of SFN. RNA sequencing and qPCR analyses were conducted to identify transcriptional changes associated with the UPR pathway. Western blot and immunofluorescence were used to assess the expression and subcellular localization of key ER stress-related proteins. A CHOP knockdown model was employed to examine the functional role of CHOP in SFN-induced apoptosis. Additionally, normal human astrocytes (HA) were used to evaluate the selectivity of SFN's cytotoxicity. In vivo validation was performed using an intracranial glioma xenograft mouse model.
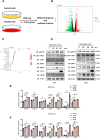
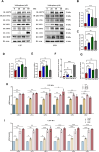
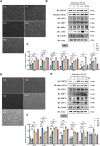
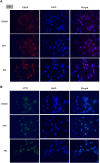
Results: SFN significantly induced apoptotic cell death in GBM cells. Mechanistically, SFN activated multiple branches of the UPR, notably increasing the expression and nuclear translocation of ATF4 and CHOP. CHOP knockdown markedly attenuated SFN-induced apoptosis. RNA-seq and KEGG enrichment analyses confirmed the involvement of the ER stress pathway. Treatment with 4-phenylbutyrate (4-PBA) suppressed SFN-induced cytotoxicity, further supporting ER stress-mediated apoptosis. In vivo, SFN reduced tumor burden and upregulated ER stress markers in intracranial tumor tissues. Importantly, SFN had minimal cytotoxic effects on normal astrocytes, suggesting a favorable therapeutic window.
Conclusions: This study demonstrates that SFN induces GBM cell apoptosis via activation of the UPR pathway, particularly through the ATF4-CHOP axis. These findings support the potential of SFN as a promising therapeutic agent for glioblastoma.
Keywords: Cleaved Caspase-3; Endoplasmic reticulum stress; Glioblastoma; In vivo model; Sulforaphane; Unfolded protein response.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Adhering to the ethical standards set forth in the Declaration of Helsinki, this research was conducted with the approval of the Ethics Committee at the First Affiliated Hospital of the University of Science and Technology of China (Anhui Provincial Hospital), under the assigned approval number 2022-KY261. Before the procedures, patients were informed of the details and provided their consent. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR, Gottardo N, Gutmann DH, Hargrave D, Holland EC, Jones DTW, Joyce JA, Kearns P, Kieran MW, Mellinghoff IK, Merchant M, Pfister SM, Pollard SM, Ramaswamy V, Rich JN, Robinson GW, Rowitch DH, Sampson JH, Taylor MD, Workman P, Gilbertson RJ. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–20. - DOI - PMC - PubMed
-
- Baralić K, Živanović J, Marić Đ, Bozic D, Grahovac L, Antonijević Miljaković E, Ćurčić M, Buha Djordjevic A, Bulat Z, Antonijević B, Đukić-Ćosić D. (2024). Sulforaphane-A Compound with Potential Health Benefits for Disease Prevention and Treatment: Insights from Pharmacological and Toxicological Experimental Studies. Antioxidants (Basel) 13(2). - PMC - PubMed
-
- Chanez B, Appay R, Guille A, Lagarde A, Colin C, Adelaide J, Denicolai E, Jiguet-Jiglaire C, Bequet C, Graillon T, Boissonneau S, Nanni-Metellus I, Dufour H, Figarella-Branger D, Chinot O, Tabouret E. Genomic analysis of paired IDHwt glioblastomas reveals recurrent alterations of MPDZ at relapse after radiotherapy and chemotherapy. J Neurol Sci. 2022;436:120207. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

