Unmet needs of adults living with mucopolysaccharidosis II: data from the Hunter Outcome Survey
- PMID: 40598289
- PMCID: PMC12211871
- DOI: 10.1186/s13023-024-03464-8
Unmet needs of adults living with mucopolysaccharidosis II: data from the Hunter Outcome Survey
Abstract
Background: Mucopolysaccharidosis II (MPS II) is a rare, life-limiting lysosomal storage disease caused by deficient iduronate-2-sulfatase activity. The current standard of care for MPS II is intravenous enzyme replacement therapy (ERT), which has been shown to improve somatic signs and symptoms and to increase life expectancy by approximately 12 years. This study reported on the somatic disease burden and clinical requirements of adult male patients in the Hunter Outcome Survey (ClinicalTrials.gov Identifier: NCT03292887).
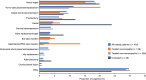
Results: Of the 373 patients in the analysis, 88 (23.6%) had cognitive impairment and 332 (89.0%) had received ERT. Almost half of all ERT-treated patients (47.0%) had undergone surgery in adulthood; the most common surgery was hernia repair (17.8% of patients). Over one-third (38.6%) reported hearing aid use. The median 6-min walk test distance for 151 treated patients was 436.0 m at the latest assessment after 18 years of age. Cardiovascular signs and symptoms were present in 71.6% (192/268) of patients and 27.3% (60/220) reported oxygen dependency after 18 years of age. Approximately half (50.9%) of ERT-treated patients experienced at least one serious adverse event in adulthood, with the most common being respiratory disorders. Intravenous ERT was well tolerated, with a rate of serious infusion-related reactions in adulthood of 0.03 per 10 patient-years.
Conclusions: Overall, adult patients with neuronopathic and non-neuronopathic MPS II had a high disease burden and requirement for surgeries, emphasizing the need to continue multidisciplinary management and regular assessments in adulthood. Further research into the differences in care needs of adult patients with MPS II is warranted. Trial registration NCT03292887 .
Keywords: Adults; Cognitive Impairment; Hunter Syndrome; Mucopolysaccharidosis type II; Neuronopathic; Non-neuronopathic.
© 2025. Takeda Pharmaceuticals International AG.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Independent Review Board/Ethics Committee approval was obtained for all participating centers. The Hunter Outcome Survey is conducted in accordance with Good Pharmacoepidemiology Practice (GPP), Good Research for Comparative Effectiveness principles, and the relevant principles of the International Conference on Harmonisation (ICH) Good Clinical Practice guidelines (ICH E6). Each patient, their parents, or a legal representative provided signed and dated written informed consent for participation in the Hunter Outcome Survey. All patient information is managed in accordance with national data protection standards. Consent for publication: Not applicable. Competing interests: J. Muenzer has received consulting fees and/or participated in advisory boards for Denali Therapeutics, JCR Pharmaceuticals, REGENXBIO, Sanofi Genzyme, and Takeda. He is a Principal Investigator for a post-trial access program for intrathecal ERT for the neuronopathic form of MPS II (sponsored by Takeda), a phase 1/2 gene editing trial for adults with MPS II (sponsored by Sangamo Therapeutics), and phase 1/2 and phase 2/3 trials of IV ERT for MPS II (sponsored by Denali Therapeutics). H. Amartino has received consulting fees, fees for service, and/or participated in advisory boards for Amicus Therapeutics, BioMarin Pharmaceutical, Janssen Pharmaceuticals, PTC Therapeutics, Sanofi Genzyme, Ultragenyx, and Takeda. He has performed contracted research for Allievex, Amicus Therapeutics, bluebird bio, JCR Pharmaceuticals, Minoryx Therapeutics, PTC Therapeutics, Sanofi Genzyme, and Takeda. R. Giugliani has received consulting fees, fees for service, speaker fees, and/or travel expenses from, and/or participated in advisory boards for Abeona Therapeutics, Alnylam Pharmaceuticals, Amicus Therapeutics, BioMarin Pharmaceutical, Chiesi Farmaceutici, Inventiva Pharma, Janssen Pharmaceuticals, JCR Pharmaceuticals, Novartis, Orphan Disease Center, Praxis Precision Medicines, PTC Therapeutics, REGENXBIO, Sanofi Genzyme, Sigilon Therapeutics, Swedish Orphan Biovitrum, Takeda, and Ultragenyx. He has performed contracted research for or received research grants from Allievex, Amicus Therapeutics, BioMarin Pharmaceutical, GC Pharma, Idorsia, Janssen Pharmaceuticals, JCR Pharmaceuticals, Lysogene, Sanofi Genzyme, Takeda, and Ultragenyx. P. Harmatz has received consulting fees/other remuneration from Aeglea, Alexion Pharmaceuticals, ArmaGen, Audentes, AVROBIO, BioMarin Pharmaceutical, Capsida Biotherapeutics, Chiesi, Denali Therapeutics, EdiGene, Enzyvant, Fondazione Telethon, Grace Science, Inventiva Pharma, JCR Pharmaceuticals, Neurogene, Novel Pharma, Orchard Therapeutics, Orphazyme, Paradigm Biopharma, PTC Therapeutics, Rallybio, REGENXBIO, Renovion, Sangamo Therapeutics, SalioGen, Sanofi Genzyme, Takeda, and Ultragenyx Pharmaceutical; and has received research support from Alexion Pharmaceuticals, Adrenas, ArmaGen, Amicus, Ascendis, ASPA, Azafaros, BioMarin Pharmaceutical, Calcilytics, Denali, Enzyvant, Homology, Inventiva Pharma, JCR, Orphazyme, Prevail, QED, RegenXbio, Sangamo Therapeutics, Swedish Orphan Biovitrum, Takeda, and Ultragenyx Pharmaceutical. S.-P. Lin has received fees for service from BioMarin Pharmaceutical, Sanofi Genzyme, and Takeda. B. Link has received fees for service and/or travel expenses from BioMarin Pharmaceutical, Chiesi Farmaceutici, Sanofi Genzyme, and Takeda. D. Molter has received fees for service and/or travel expenses from BioMarin Pharmaceutical and Takeda. U. Ramaswami has participated in advisory boards for Amicus Therapeutics, JCR Pharmaceuticals, Sanofi, and Takeda. She has received research grants from Amicus Therapeutics, IntraBio, and Takeda. M. Scarpa has received consulting fees, fees for service, and/or participated in advisory boards for Actelion Pharmaceuticals, Alexion Pharmaceuticals, BioMarin Pharmaceuticals, Chiesi Farmaceutici, Orchard Therapeutics, Orphazyme, Paradigm Biopharma, PTC Therapeutics, Sanofi Genzyme, Takeda, and Ultragenyx. He has performed contracted research for Alexion Pharmaceuticals, BioMarin Pharmaceutical, CTD, Orchard Therapeutics, Orphazyme, Paradigm Biopharmaceuticals, PTC Therapeutics, Sanofi Genzyme, and Takeda. J. Botha is a full-time employee of Takeda and stockholder of Takeda Pharmaceuticals Company Limited. J. Audi was an employee of Takeda and stockholder of Takeda Pharmaceuticals Company Limited at the time of this analysis (current affiliation is Ultragenyx Europe GmbH, Allschwil, Basel, Switzerland). B.K. Burton has received consulting fees and/or participated in advisory boards for Agios Pharmaceuticals, Alexion, Alltrna, Amgen, Aro, Applied Therapeutics, BioMarin Pharmaceutical, Chiesi Farmaceutici, JCR Pharmaceuticals, Jnana Therapeutics, Moderna, Orchard Therapeutics, Passage Bio, Sanofi Genzyme, Takeda, Travere, and Ultragenyx. She has performed contracted research for Takeda and has been involved in company-sponsored clinical trials with BioMarin Pharmaceutical, Denali Therapeutics, Homology Medicines, JCR Pharmaceuticals, Jnana Therapeutics, Sangamo Therapeutics, Synlogic, Takeda, and Ultragenyx.
Figures



References
-
- Neufeld EF, Muenzer J, et al. The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. p. 3421–52.
-
- Wraith JE, Beck M, Giugliani R, Clarke J, Martin R, Muenzer J. Initial report from the Hunter Outcome Survey. Genet Med. 2008;10:508–16. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

