Human platelet lysate standardization across three independent European blood establishments
- PMID: 40598301
- PMCID: PMC12210671
- DOI: 10.1186/s13287-025-04445-9
Human platelet lysate standardization across three independent European blood establishments
Abstract
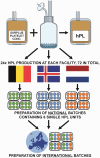
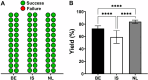
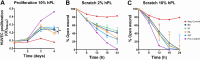
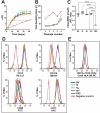
Human platelet lysate (hPL) is a clinically safe alternative to fetal bovine serum (FBS). However, variability in blood donation practices, platelet concentrate preparation methods, storage and hPL manufacturing complicates standardization across jurisdictions. This study aimed to establish a first multinational hPL manufacturing standardization across three European blood centers to test feasibility and variability. A single batch of hPL production sets was distributed to the participating centers. There, hPL was produced following a single standard operating protocol but starting from each center's unique platelet concentrates. Each center prepared four 'national' hPL batches and four 'international' batches. Researchers conducted blinded quality and variation analyses to ensure unbiased results. All hPL batches exhibited comparable total protein levels, pH, ionic strength, and lactate content. Analysis of twelve growth factors showed minor variations across batches. Endothelial cell outgrowth and wound closure were slower in hPL than FBS but remained consistent across batches. Mesenchymal stem cell (MSC) doubling was significantly faster in hPL than in FBS, with MSC phenotype consistency confirmed via flow cytometry. Differentiation into adipogenic and osteogenic tissue was successful in all hPL samples. The inter-institutional variation across all national batches was higher for all critical outcome parameters compared to the variation in the four international batches. These findings confirm the feasibility of manufacturing standardized hPL across borders and show lower variability when doing so. This supports further efforts to stabilize hPL supply and advance cytotherapy standardization in Europe.
Keywords: Culture media; Human platelet lysate; Manufacturing; Mesenchymal stem cells; Standardization; Tissue culture.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Material was obtained from consenting healthy voluntary blood donors. The project was entitled ‘Adviesaanvraag voor biobank Rode Kruis Vlaanderen, Versie 3, Amendement 3’ approved by the Ethics Institutional Review Board named Universitair Ziekenhuis Leuven with number S62549, dd 22/08/2023. Cells obtained from Lonza (material number PT-5006, batch number 22TL213997) and PromoCell (material number C-12208, batch number 505Z011) were isolated from donated human tissue after obtaining permission for their use in research applications by informed consent or legal authorization. Competing interests: WD, PV and HBF are listed as inventors of pending patent applications for producing platelet lysates. All authors are employed by the Belgian Red Cross-Flanders.
Figures



References
-
- Palombella S, Perucca Orfei C, Castellini G, Gianola S, Lopa S, Mastrogiacomo M, et al. Systematic review and meta-analysis on the use of human platelet lysate for mesenchymal stem cell cultures: comparison with fetal bovine serum and considerations on the production protocol. Stem Cell Res Ther. 2022;13(1):142. - DOI - PMC - PubMed
-
- Erickson GA, Bolin SR, Landgraf JG. Viral contamination of fetal bovine serum used for tissue culture: risks and concerns. Dev Biol Stand. 1991;75:173–5. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

