Extensive data mining uncovers novel diversity among members of the rare biosphere within the Thermoplasmatota
- PMID: 40598319
- PMCID: PMC12220078
- DOI: 10.1186/s40168-025-02140-8
Extensive data mining uncovers novel diversity among members of the rare biosphere within the Thermoplasmatota
Abstract
Background: Rare species, especially of the marine sedimentary biosphere, have long been overlooked owing to the complexity of sediment microbial communities, their sporadic temporal and patchy spatial abundance, and challenges in cultivating environmental microorganisms. In this study, we combined enrichments, targeted metagenomic sequencing, and extensive data mining to uncover uncultivated members of the archaeal rare biosphere in marine sediments.
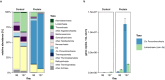
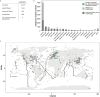
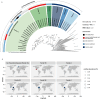
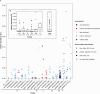
Results: In protein-amended enrichments, we detected the ecologically and metabolically uncharacterized class Candidatus Penumbrarchaeia within the phylum Thermoplasmatota. By screening more than 8000 metagenomic runs and 11,479 published genome assemblies, we expanded the phylogeny of Ca. Penumbrarchaeia by 3 novel orders. All six identified families of this class show low abundance in environmental samples characteristic of rare biosphere members. Members of the class Ca. Penumbrarchaeia were predicted to be involved in organic matter degradation in anoxic, carbon-rich habitats. All Ca. Penumbrarchaeia families contain high numbers of taxon-specific orthologous genes, highlighting their environmental adaptations and habitat specificity. Besides, members of this group exhibit the highest proportion of unknown genes within the entire phylum Thermoplasmatota, suggesting a high degree of functional novelty in this class.
Conclusions: In this study, we emphasize the necessity of targeted, data-integrative approaches to deepen our understanding of the rare biosphere and uncover the functions and metabolic potential hidden within these understudied taxa. Video Abstract.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Evidence of microbial reductive dehalogenation in deep-sea cold seeps and its implications for biogeochemical cycles.Microbiome. 2025 Jul 2;13(1):156. doi: 10.1186/s40168-025-02147-1. Microbiome. 2025. PMID: 40605092 Free PMC article.
-
Environmental diversity of Candidatus Babelota and their relationships with protists.mSystems. 2025 Jun 17;10(6):e0026125. doi: 10.1128/msystems.00261-25. Epub 2025 May 28. mSystems. 2025. PMID: 40434078 Free PMC article.
-
Structure and Function Features of Abundant and Rare Prokaryotic Communities Along Nearshore to Offshore Transitions.Environ Microbiol. 2025 Jul;27(7):e70144. doi: 10.1111/1462-2920.70144. Environ Microbiol. 2025. PMID: 40624949
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
References
-
- Pascoal F, Costa R, Magalhães C. The microbial rare biosphere: current concepts, methods and ecological principles. FEMS Microbiol Ecol. 2020;97(1): fiaa227. 10.1093/femsec/fiaa227. - PubMed
-
- Rabinowitz D, Rapp JK, Dixon PM. Competitive abilities of sparse grass species: means of persistence or cause of abundance. Ecology. 1984;65(4):1144–54. 10.2307/1938322.
-
- Rabinowitz D. Seven forms of rarity. In: Synge H, editor. The biological aspects of rare plant conservation. New York: John Wiley and Sons; 1981. p. 205–17.
MeSH terms
Substances
Grants and funding
- EXC-2077-390741603/Deutsche Forschungsgemeinschaft
- EXC-2077-390741603/Deutsche Forschungsgemeinschaft
- XJ2300006031/Start-up research fund of Hainan University, China
- LT0050/2023-L/Human Frontier Science Program
- 205320_215395/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
LinkOut - more resources
Full Text Sources

