Interleukin-1 receptor antagonist overexpression in mesenchymal stem cells improves hemorrhagic cystitis outcomes via HtrA serine peptidase 3
- PMID: 40598340
- PMCID: PMC12217520
- DOI: 10.1186/s13287-025-04443-x
Interleukin-1 receptor antagonist overexpression in mesenchymal stem cells improves hemorrhagic cystitis outcomes via HtrA serine peptidase 3
Erratum in
-
Correction: Interleukin-1 receptor antagonist overexpression in mesenchymal stem cells improves hemorrhagic cystitis outcomes via HtrA serine peptidase 3.Stem Cell Res Ther. 2025 Jul 7;16(1):353. doi: 10.1186/s13287-025-04494-0. Stem Cell Res Ther. 2025. PMID: 40624703 Free PMC article. No abstract available.
Abstract
Background: Hemorrhagic cystitis (HC), a frequent complication of hematopoietic stem cell transplantation (HSCT), significantly affects quality of life and may worsen prognosis. Mesenchymal stem cells (MSCs) are known for their anti-inflammatory and tissue-regenerative properties. IL-1 receptor antagonist (IL-1Ra) blocks IL-1α and IL-1β by binding IL-1 receptors, offering potential therapeutic benefits. The aim of this study was to explore the therapeutic effect of MSCs overexpressing IL-1Ra on HC and investigate the underlying mechanisms.
Methods: MSCs were isolated from human umbilical cord tissues, and IL-1Ra-overexpressing MSCs (oeIL-1Ra-MSCs) were generated using lentiviral transfections. HC was induced in rats by cyclophosphamide administration. Rats received tail vein injections of either oeIL-1Ra-MSCs or control MSCs (Mock-MSCs). Hematuria and bladder tissue changes were assessed using test strips and hematoxylin & eosin (HE) staining. Immunohistochemistry detected molecular changes in bladder tissues. Gene expression differences between the two MSC groups were analyzed by mRNA sequencing and ChIP techniques.
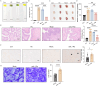
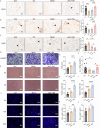
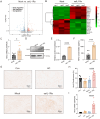
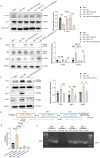
Results: Treatment with oeIL-1Ra-MSCs significantly alleviated hematuria and reduced bladder edema and hemorrhage, and reduced mRNA expression levels of IL-1β, IL-6, and TNF-α in bladder tissues, compared with those in the Mock-MSC treatment group. Immunohistochemical staining showed a higher presence of CD105-positive cells (a marker for human MSCs) and CD31-positive vessels in bladder tissues treated with oeIL-1Ra-MSCs, indicating enhanced MSC migration and vascular stability. In vitro migration assay demonstrated a higher migration capacity of IL-1Ra overexpressing MSCs compared with that of control MSCs. Moreover, angiopoietin-1 (Ang-1) expression increased, while Angiopoietin-2 (Ang-2) expression decreased in bladder tissues treated with oeIL-1Ra-MSCs, suggesting enhanced blood vessel stabilization. Conditioned medium from oeIL-1Ra-MSC cultures stimulated human umbilical vein endothelial cell (hUVEC) migration, proliferation, and angiogenesis more effectively compared with that in control MSCs. mRNA sequencing revealed elevated HtrA3 expression in oeIL-1Ra-MSCs compared with that in control MSCs. Molecular analysis suggested that IL-1Ra overexpression in MSCs upregulated HtrA3 expression through inhibition of the JNK-c-Jun pathway and activation of the ERK-Egr-1 pathway.
Conclusion: Overexpression of IL-1Ra significantly enhances the therapeutic efficacy of MSCs in HC by promoting MSC migration to damaged bladder tissues, suppressing inflammation, stabilizing blood vessels, and upregulating angiogenesis via activation of HtrA3 signaling pathways.
Keywords: Hemorrhagic cystitis; HtrA serine peptidase 3; Interleukin-1 receptor antagonist; Mesenchymal stem cell; Migration.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The umbilical cord was obtained from the Department of Obstetrics at the Second Hospital of Shandong University. The source of human umbilical cord and animal experiments were approved by the Ethics Committee of the Second Hospital of Shandong University. Project title: Efficacy and mechanism of overexpression of IL-1Ra mesenchymal stem cells and their exosomes in the treatment of hemorrhagic cystitis; No. 2024SCR003; date of approval: 2024-08-12. All human umbilical cord tissues used in this study received written informed consent from the parturient prior to sample collection. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Human umbilical cord mesenchymal stem cells-derived extracellular vesicles as a therapeutic approach to ameliorate bladder injury in animal models of radiation cystitis.Stem Cell Res Ther. 2025 Jul 18;16(1):387. doi: 10.1186/s13287-025-04503-2. Stem Cell Res Ther. 2025. PMID: 40682138 Free PMC article.
-
Cell Sheets Formation Enhances Therapeutic Effects of Human Umbilical Cord Mesenchymal Stem Cells on Spinal Cord Injury.CNS Neurosci Ther. 2024 Dec;30(12):e70163. doi: 10.1111/cns.70163. CNS Neurosci Ther. 2024. PMID: 39670537 Free PMC article.
-
Human Infrapatellar Fat Pad Mesenchymal Stem Cell-derived Extracellular Vesicles Purified by Anion Exchange Chromatography Suppress Osteoarthritis Progression in a Mouse Model.Clin Orthop Relat Res. 2024 Jul 1;482(7):1246-1262. doi: 10.1097/CORR.0000000000003067. Epub 2024 Apr 19. Clin Orthop Relat Res. 2024. PMID: 38662932 Free PMC article.
-
Mesenchymal stem cells transplantation for perianal fistulas: a systematic review and meta-analysis of clinical trials.Stem Cell Res Ther. 2023 Apr 26;14(1):103. doi: 10.1186/s13287-023-03331-6. Stem Cell Res Ther. 2023. PMID: 37101285 Free PMC article.
-
Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis.Cochrane Database Syst Rev. 2021 Oct 25;10(10):CD013650. doi: 10.1002/14651858.CD013650.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693515 Free PMC article.
References
-
- Ruggeri A, Roth-Guepin G, Battipaglia G, Mamez AC, Malard F, Gomez A, et al. Incidence and risk factors for hemorrhagic cystitis in unmanipulated haploidentical transplant recipients. Transpl Infect Dis. 2015;17(6):822–30. - PubMed
-
- El-Zimaity M, Saliba R, Chan K, Shahjahan M, Carrasco A, Khorshid O, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation: donor type matters. Blood. 2004;103(12):4674–80. - PubMed
-
- Linder BJ, Tarrell RF, Boorjian SA. Cystectomy for refractory hemorrhagic cystitis: contemporary etiology, presentation and outcomes. J Urol. 2014;192(6):1687–92. - PubMed
-
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–40. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

