Tensile stress promotes the chondrogenic ability of condylar cartilage stem/progenitor cells in the temporomandibular joint via the Piezo1-Ca2+-Prkca pathway
- PMID: 40598380
- PMCID: PMC12210528
- DOI: 10.1186/s13287-025-04439-7
Tensile stress promotes the chondrogenic ability of condylar cartilage stem/progenitor cells in the temporomandibular joint via the Piezo1-Ca2+-Prkca pathway
Abstract
Background: Tensile force is a key regulator for condylar cartilage remodeling in the temporomandibular joint (TMJ), and this biomechanical characteristic underlies the mechanisms of mandibular growth modification in orthodontic practice. Cartilage stem/progenitor cells (CSPCs) in the superficial layer of condylar cartilage play an essential part in the development and remodeling of condylar cartilage. However, the regulatory role of tensile force on condylar CSPCs remains unclear. This study aimed to investigate the impact of tensile loading on condylar CSPCs and explore the molecular mechanisms within.
Methods: The mandibular advancement (MA) model was constructed to apply tensile force on the condylar cartilage in vivo. Flow cytometry and transcriptome sequencing were utilized to assess the percentage of CSPCs and gene expression in the superficial layer of rat condylar cartilage. Lineage tracing with cathepsin K (Ctsk) in mice was employed to trace the differentiation of CSPCs. 10% equibiaxial dynamic strain was loaded on rat CSPCs for cell stretching in vitro. GsMTx4 was used to inhibit the Piezo1 channel, and the calcium chelating agent BAPTA was used to block the Ca2+ influx of rat CSPCs. siRNA was applied to knock down the protein kinase C alpha (Prkca) of rat CSPCs in vitro and in vivo.
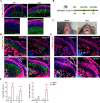
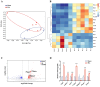
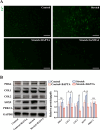
Results: Cartilage thickening and a transient reduction of the CSPCs proportion in the superficial layer of the condylar cartilage were observed after 1 week of MA. The ratio of Ctsk and type II collagen double-positive cells climbed in the first week after MA, and 2 weeks later, the ratio of Ctsk and EdU double-positive cells rose. The expression level of chondrogenic-related genes, Piezo1, and Prkca was elevated in CSPCs after tensile loading. GsMTx4 and BAPTA could block the Ca2+ influx into CSPCs caused by tensile stress. Furthermore, BAPTA and siPrkca could inhibit the stretch-induced chondrogenesis of CSPCs.
Conclusions: We uncovered that tensile stress could cause a transient shrinkage of the CSPCs pool in condylar cartilage, resulting from the accelerated chondrogenesis of CSPCs. Tensile force could promote the chondrogenic ability of CSPCs via the Piezo1-Ca2+-Prkca pathway. This study suggested a new regulatory route for mandibular growth modification in orthodontic practice.
Keywords: Cartilage; Cartilage stem/progenitor cells; Mandibular advancement; Mandibular condyle; Mechanical stress; Temporomandibular joint.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All the procedures of animal experiments were approved by the Animal Welfare Committee of the School and Hospital of Stomatology, Tongji University, under no. 2024-DW-31. The title of the approved project was “Research on the identification of mechanical force-responsive subpopulation in mouse fibrocartilage stem cells and the underlying mechanisms”. The date of approval was February 22, 2024. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures




Similar articles
-
Piezo1 regulates fibrocartilage stem cell in cartilage growth and osteoarthritis.Osteoarthritis Cartilage. 2025 Aug;33(8):980-991. doi: 10.1016/j.joca.2025.04.013. Epub 2025 May 8. Osteoarthritis Cartilage. 2025. PMID: 40345612
-
PTH Promotes Chondrogenesis of Fibrocartilage Stem Cells and Alleviates Temporomandibular Joint Osteoarthritis.Tissue Eng Regen Med. 2025 Jul;22(5):705-718. doi: 10.1007/s13770-025-00723-y. Epub 2025 Jun 16. Tissue Eng Regen Med. 2025. PMID: 40522441 Free PMC article.
-
Effects of estrogen and mechanical loading on cultured cells derived from mandibular condylar cartilage.Sci Rep. 2025 Jul 2;15(1):23470. doi: 10.1038/s41598-025-07770-4. Sci Rep. 2025. PMID: 40603527 Free PMC article.
-
The impact of dietary consistency on structural craniofacial components: Temporomandibular joint/condyle, condylar cartilage, alveolar bone and periodontal ligament. A systematic review and meta-analysis in experimental in vivo research.Arch Oral Biol. 2018 Oct;94:33-47. doi: 10.1016/j.archoralbio.2018.06.016. Epub 2018 Jun 19. Arch Oral Biol. 2018. PMID: 29957455
-
Effect of mechanical loading on the metabolic activity of cells in the temporomandibular joint: a systematic review.Clin Oral Investig. 2018 Jan;22(1):57-67. doi: 10.1007/s00784-017-2189-9. Epub 2017 Aug 1. Clin Oral Investig. 2018. PMID: 28761983 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

