Diagnostic value of the wnt target and cancer-associated blood biomarker hPG80: ONCOPRO case-control prospective study
- PMID: 40598473
- PMCID: PMC12218953
- DOI: 10.1186/s40364-025-00793-z
Diagnostic value of the wnt target and cancer-associated blood biomarker hPG80: ONCOPRO case-control prospective study
Abstract
Background: hPG80 (circulating progastrin), initially recognized for its oncogenic properties due to its direct link to the Wnt signaling pathway, is secreted by cancer cells and detectable in the blood of cancer patients. The ONCOPRO centralized case-control study (NCT03787056) was designed to prospectively evaluate the diagnostic utility of hPG80 in patients with 16 different types of cancer.
Methods: hPG80 levels were measured in 421 patients with 16 newly diagnosed cancers (median age 65.6 years old) using the DxPG80.Lab kit (Biodena Care). The diagnostic performance of hPG80 (the primary endpoint) was assessed by comparing baseline hPG80 levels in cancer patients with those of 330 asymptomatic aged-matched healthy subjects from the general population.
Results: Between 2018 and 2022, a total of 506 cancer patients were enrolled in the study, with 421 assessable across 16 distinct cancer cohorts. hPG80 concentrations were significantly higher in cancer patients compared to the healthy population (median 3.8 [IQR: 1.0-11.1] vs. 1.9 [IQR: 0.6-4.2] pM, P < 0.0001). hPG80 levels were not impacted by renal failure, liver dysfunction, or cancer-associated inflammation. The diagnostic accuracy in cancer patients was ROC AUC 0.63, 95% CI = [0.59-0.67]. The highest diagnostic accuracy was seen in patients with lung cancer (AUC 0.75, 95% CI [0.68-0.82]; specificity = 88% for hPG80 > 7.73 pM in patients aged > 58 years old) and hepatocellular carcinoma HCC (ROC AUC 0.75, 95% CI [0.66-083]; specificity = 88% for hPG80 > 7.73 pM in patients aged > 58 years old).
Conclusions: This large prospective study confirms that cancer patients have significantly higher hPG80 blood concentration compared to the healthy population. Incorporating this straightforward ELISA assay into screening programs is warranted.
Trial registration: NCT03787056.
Keywords: Biomarkers, tumor; Early detection of Cancer; Prospective studies; Wnt signaling pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics and consent to participate declarations: All patients gave their consent and signed the informed consent. The protocol, the written information sheet and the consent form for the study obtained a favorable opinion from to ethics committee Ile de France VI on October 3rd 2018. This notification was transmitted to the French health authority ANSM. All Protocol amendments were approved by the same ethic committee. Consent for publication: Not applicable. Competing interests: DJ, AP, and BV are employed by Biodena Care (Progastrin Manufacturing). All the other coauthors have no conflict of interest to disclose.
Figures


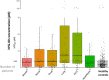

References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

