Development of a novel ddPCR assay for the simultaneous detection of the protozoan parasites Leishmania infantum and Leishmania tarentolae
- PMID: 40598545
- PMCID: PMC12210423
- DOI: 10.1186/s13071-025-06871-3
Development of a novel ddPCR assay for the simultaneous detection of the protozoan parasites Leishmania infantum and Leishmania tarentolae
Abstract
Background: Leishmaniases, caused by protozoan parasites of the genus Leishmania, are vector-borne diseases occurring mainly in the tropics and subtropics of the world, as well as in the Mediterranean Basin. In this area, the mammalian pathogen Leishmania infantum is endemic, along with the reptile-associated Leishmania tarentolae. The two species occur in sympatry, and there is evidence that the exposure to L. tarentolae in mammalian hosts may elicit a protective immune response towards pathogenic Leishmania species. Accurate detection methods for both species are therefore crucial for gathering comprehensive information on the epidemiology of leishmaniases. In microbiological diagnosis, limits in detection performance imply the risk of false negatives and other issues, which highlights the need for sensitive methods.
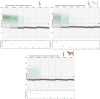
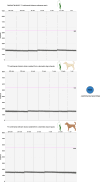
Methods: Here, we developed a droplet digital polymerase chain reaction assay targeting the kinetoplast minicircle DNA, for the simultaneous and differential detection of L. infantum and L. tarentolae. The assay features primers designed to bind to both species and species-specific probes. The assay was validated on three cultured isolates for each species, whose cells were spiked into Leishmania-negative dog blood, and on Leishmania-positive sand flies. Sensitivity was assessed with testing serial dilutions, and specificity was evaluated by assessing the cross-reactivity of the probes with the controls of Leishmania-free dog blood and male sand fly DNA.
Results: The assay demonstrated high sensitivity, with a limit of detection corresponding to one Leishmania cell in the reaction mix for isolates of both L. infantum and L. tarentolae. Limited cross-reaction of the L. tarentolae-targeting probe was observed on L. infantum isolates. No cross-reaction was observed with the controls of Leishmania-free dog blood and male sand flies.
Conclusions: The protocol can represent a valuable method for comprehensive surveillance in both canine hosts and sand flies in areas in which L. infantum and L. tarentolae occur in sympatry.
Keywords: Leishmania infantum; Leishmania tarentolae; Droplet digital PCR; Epidemiology; Leishmaniasis; Sand flies.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The dog blood used for the presented research was obtained from a previous study. [30]. The methods to handle dog blood were approved by the Clinvet Institutional Animal Care and Use Committee (n° CG1331-CVMO22/216). Consent for publication: Not applicable. Competing interests: S.E. is an Associate Editor for Parasites & Vectors, and A.M. is employed by VisMederi Srl. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship.
Figures