Palazestrant, a novel oral Complete Estrogen Receptor Antagonist (CERAN) and Selective Estrogen Receptor Degrader (SERD), in patients with ER+/HER2- advanced or metastatic breast cancer: phase 1/2 study results
- PMID: 40598566
- PMCID: PMC12210654
- DOI: 10.1186/s13058-025-02049-y
Palazestrant, a novel oral Complete Estrogen Receptor Antagonist (CERAN) and Selective Estrogen Receptor Degrader (SERD), in patients with ER+/HER2- advanced or metastatic breast cancer: phase 1/2 study results
Abstract
Background: Endocrine resistance is a major challenge in treating patients with ER+ /HER2- metastatic breast cancer (MBC) necessitating a switch from endocrine therapy to more toxic therapies. Mutations in ESR1 constitute a key mechanism of resistance to endocrine therapy in ER+ /HER2- BC. Therapies that overcome endocrine resistance are needed. Palazestrant is a novel oral complete estrogen receptor (ER) antagonist (CERAN) and selective ER degrader (SERD) belonging to a new class of ER-targeting agents that completely blocks estrogen-induced transcriptional activity, regardless of ESR1 mutation status. This first-in-human, open-label, multicenter, phase 1/2 dose-escalation/expansion study was designed to determine the recommended phase 2 dose (RP2D) and to evaluate safety, pharmacokinetics, and antitumor activity of palazestrant in patients with ER+ /HER2- MBC with disease progression on prior treatment.
Methods: Adults with ER+ /HER2‒ MBC who received ≥ 1 prior line of endocrine therapy for advanced disease and ≤ 2 prior chemotherapy regimens for metastatic disease were eligible. Patients received once-daily oral palazestrant (30-300 mg) in 28-day cycles until progression or intolerable toxicity.
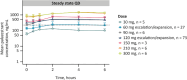
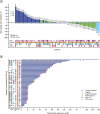
Results: This study enrolled 146 patients. No dose-limiting toxicities were observed at doses up to 300 mg/day palazestrant. Confirmed partial responses were observed with 60 and 120 mg/day palazestrant. Both doses showed similar and tolerable safety profiles, favorable pharmacokinetics, and steady-state plasma concentrations above the predicted threshold for complete ER inhibition. Greater clinical benefit at palazestrant 120 mg/day (46%) versus 60 mg/day (19%) led to selection of 120 mg/day as RP2D and study expansion dose. At 120 mg/day, the median progression-free survival was 4.8 months (95% CI, 3.5-7.1) overall and 5.6 months (95% CI, 4.8-NE) among patients with cancers with ESR1 mutations. Most treatment-emergent adverse events (TEAEs) were grade 1-2. The most common TEAEs were nausea (62.8%), vomiting (29.1%), and fatigue (25.6%). The most common grade ≥ 3 TEAE was transient neutropenia (10.5%) managed by dose interruption and reduction.
Conclusions: Palazestrant demonstrated a manageable safety profile, with antitumor activity observed in patients with heavily pretreated cancers with wild-type and ESR1-mutated BC. These data support the ongoing phase 3 study evaluating palazestrant in patients with ER+ /HER2 - MBC.
Trial registration: ClinicalTrials.gov, NCT04505826 . Registered August 6, 2020.
Keywords: Complete estrogen receptor antagonist; Endocrine therapy; Estrogen receptor mutation; Estrogen receptor-positive human epidermal growth factor receptor 2-negative metastatic breast cancer; Palazestrant; Selective estrogen receptor degrader.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Council on Harmonization of Technical Requirements for Pharmaceuticals for Human Use – Good Clinical Practice (ICH-GCP) guidelines, and all applicable local and national (US Food and Drug Administration [FDA]) regulatory requirements. All patients provided written informed consent prior to participation in the study. Competing interests: Erika P. Hamilton has received research support paid to her institution from AbbVie, Acerta Pharma, Accutar Biotech, ADC Therapeutics, Akesobio Australia, Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, Atlas Medx, BeiGene, Black Diamond, Bliss Biopharmaceutical, Boehringer Ingelheim, Bristol-Myers Squibb, Cascadian Therapeutics, Clovis, Compugen, Context Therapeutics, Cullinan, Curis, CytomX, Daiichi Sankyo, Dana Farber Cancer Institute, Dantari, Deciphera, Duality Biologics, eFFECTOR Therapeutics, Eisai, Ellipses Pharma, Elucida Oncology, EMD Serono, Fochon Pharmaceuticals, FujiFilm, G1 Therapeutics, Gilead Sciences, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, Inspirna, InventisBio, Jacobio, Karyopharm, K-Group Beta, Kind Pharmaceuticals, Leap Therapeutics, Lilly, Loxo Oncology, Lycera, MabSpace Biosciences, MacroGenics, MedImmune, Mersana, Merus, Millennium, Molecular Templates, Myriad Genetic Laboratories, Novartis, NuCana, Olema, Oncomed, Oncothyreon, ORIC Pharmaceuticals, Orinove, Orum Therapeutics, Pfizer, PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxikon, Prelude Therapeutics, Profound Bio, Radius Health, Regeneron, Relay Therapeutics, Repertoire Immune Medicine, Rgenix, Roche/Genentech, Seagen, Sermonix Pharmaceuticals, Shattuck Labs, Silverback Therapeutics, Stemcentrx, Stemline Therapeutics, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem Oncology, Zenith Epigenetics, and Zymeworks. She has also acted as a consultant/advisor and received payments to her institution from Accutar Biotechnology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos Pharmaceuticals, Fosun Pharma, Gilead Sciences, Jazz Pharmaceuticals, Jefferies LLC, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Pfizer, Roche/Genentech, Stemline Therapeutics, Tempus Labs, Theratechnologies, Tubulis, Verascity Science, and Zentalis Pharmaceuticals. Manish R. Patel has acted in a leadership role for ION Pharma, has received honoraria from Janssen Oncology, has acted in a consulting or advisory role for Accutar Biotech, Daiichi Sankyo/UCB Japan, Kura Oncology, Nurix, and Olema Oncology, and has received research support paid to his institution from Acerta Pharma, ADC Therapeutics, Agenus, Allorio Therapeutics, Artios, AstraZeneca, BioNTech AG, BioTheryX, Boehringer Ingelheim, Bristol-Myers Squibb/Celgene, Compugen, Conjupro Biotherapeutics, Cullinan Oncology, Cyteir Therapeutics, Daiichi Sankyo, Erasca, Inc., Genentech/Roche, Gergiamune, Gilead Sciences, GlaxoSmithKline, H3 Biomedicine, Hengrui Therapeutics, Hotspot Therapeutics, Hutchison MediPharma, Immune-Onc Therapeutics, Immunitas, Incyte, Janssen, Kineta, Klus Pharma, Kura Oncology, Kymab, Lilly, Loxo, LSK Biopartners, Mabspace, Macrogenics, Merck, Millenium, Moderna Therapeutics, Novartis, Nurix, ORIC Pharmaceuticals, Pfizer, Pionyr, Prelude Therapeutics, Puretech, Ribon Therapeutics, Seven and Eight Biopharmaceuticals, Step Pharma, Syndax, Taiho Pharmaceutical, Tesaro, and Vividon Therapeutics. Virginia F. Borges has acted in a consulting or advisory role for AstraZeneca, Gilead, Pfizer, and Seagen, has received research support paid to her institution from AstraZeneca, Gilead, Olema, Pfizer, and Seagen, and owns stocks in Pearl Scientific. Jane L. Meisel has acted in a consulting or advisory role for AstraZeneca, Clovis, Genentech, GSK, Novartis, Olema, Pfizer, Puma, and Seagen, and has received research support paid to her institution from AstraZeneca, Olema, Pfizer, Seagen, and Sermonix. Meena Okera has no conflicts of interest to disclose relating to the work. Carlos A. Alemany has received research support paid to his institution from Bayer, Bristol-Myers Squibb, Exelixis, Janssen, Johnson & Johnson, Merck, Novartis, Olema Oncology, Pfizer, and Seagen. Timothy J. Pluard has received research support paid to his institution from Pfizer, Novartis, Gilead, Stemline, AstraZeneca, Arvinas, Scorpion Therapeutics, H3Biomedicine, Olema Pharmaceuticals, Dantari, Daiichi, Sanofii, Jazz Pharmaceuticals, Carrick Therapeutics, Daiichi, Merck, Sermoix, and has acted in a consulting or advisory role for Pfizer, AstraZeneca, Arvinas, Gilead, Jazz. Speaking: Gilead, Stemline, AstraZeneca. Robert Wesolowski has acted as an Advisory Board member and Scientific Steering Committee member for Celcuity, and has served on an Advisory Board for Pfizer. Dhanusha Sabanathan has no conflicts of interest to disclose relating to the work, aside from acknowledging the sponsor of the study (Olema Oncology). Kathy D. Miller has no conflicts of interest to disclose relating to the work, aside from acknowledging the sponsor of the study (Olema Oncology). Alison K. Conlin has acted in a consulting or advisory role for AstraZeneca, Gilead Sciences, and Seattle Genetics/Astellas, and has received payment for travel/accommodation/expenses from Seattle Genetics/Astellas. Nicole McCarthy has acted in a consulting or advisory role for AstraZeneca, Gilead Sciences, Merck, and Novartis, and has received a travel grant from Novartis. Margaret Tonda and Mark Shilkrut are employees of and own stocks in Olema Oncology. Nancy U. Lin has received research support paid to her institution from Genentech, Pfizer, Merck, Seattle Genetics, Zion Pharmaceuticals, Olema Oncology, and AstraZeneca. She has received honoraria for consulting work from Puma, Seattle Genetics, Daiichi-Sankyo, AstraZeneca, Denali Therapeutics, Prelude Therapeutics, Olema Oncology, Aleta BioPharma, Affinia Therapeutics, Voyager Therapeutics, Janssen, Blueprint Medicines, Stemline/Menarini, Artera Inc., and Reverie Labs. Manish R. Patel has acted in a leadership role for ION Pharma, has received honoraria from Janssen Oncology, has acted in a consulting or advisory role for Accutar Biotech, Daiichi Sankyo/UCB Japan, Kura Oncology, Nurix, and Olema Oncology, and has received research support paid to his institution from Acerta Pharma, ADC Therapeutics, Agenus, Allorio Therapeutics, Artios, AstraZeneca, BioNTech AG, BioTheryX, Boehringer Ingelheim, Bristol-Myers Squibb/Celgene, Compugen, Conjupro Biotherapeutics, Cullinan Oncology, Cyteir Therapeutics, Daiichi Sankyo, Erasca, Inc., Genentech/Roche, Gergiamune, Gilead Sciences, GlaxoSmithKline, H3 Biomedicine, Hengrui Therapeutics, Hotspot Therapeutics, Hutchison MediPharma, Immune-Onc Therapeutics, Immunitas, Incyte, Janssen, Kineta, Klus Pharma, Kura Oncology, Kymab, Lilly, Loxo, LSK Biopartners, Mabspace, Macrogenics, Merck, Millenium, Moderna Therapeutics, Novartis, Nurix, ORIC Pharmaceuticals, Pfizer, Pionyr, Prelude Therapeutics, Puretech, Ribon Therapeutics, Seven and Eight Biopharmaceuticals, Step Pharma, Syndax, Taiho Pharmaceutical, Tesaro, and Vividon Therapeutics. Virginia F. Borges has acted in a consulting or advisory role for AstraZeneca, Gilead, Pfizer, and Seagen, has received research support paid to her institution from AstraZeneca, Gilead, Olema, Pfizer, and Seagen, and owns stocks in Pearl Scientific. Jane L. Meisel has acted in a consulting or advisory role for AstraZeneca, Clovis, Genentech, GSK, Novartis, Olema, Pfizer, Puma, and Seagen, and has received research support paid to her institution from AstraZeneca, Olema, Pfizer, Seagen, and Sermonix. Meena Okera has no conflicts of interest to disclose relating to the work. Carlos A. Alemany has received research support paid to his institution from Bayer, Bristol-Myers Squibb, Exelixis, Janssen, Johnson & Johnson, Merck, Novartis, Olema Oncology, Pfizer, and Seagen. Timothy J. Pluard has received research support paid to his institution from Pfizer, Novartis, Gilead, Stemline, AstraZeneca, Arvinas, Scorpion Therapeutics, H3Biomedicine, Olema Pharmaceuticals, Dantari, Daiichi, Sanofii, Jazz Pharmaceuticals, Carrick Therapeutics, Daiichi, Merck, Sermoix, and has acted in a consulting or advisory role for Pfizer, AstraZeneca, Arvinas, Gilead, Jazz. Speaking: Gilead, Stemline, AstraZeneca. Robert Wesolowski has acted as an Advisory Board member and Scientific Steering Committee member for Celcuity, and has served on an Advisory Board for Pfizer. Dhanusha Sabanathan has no conflicts of interest to disclose relating to the work, aside from acknowledging the sponsor of the study (Olema Oncology). Kathy D. Miller has no conflicts of interest to disclose relating to the work, aside from acknowledging the sponsor of the study (Olema Oncology). Alison K. Conlin has acted in a consulting or advisory role for AstraZeneca, Gilead Sciences, and Seattle Genetics/Astellas, and has received payment for travel/accommodation/expenses from Seattle Genetics/Astellas. Nicole McCarthy has acted in a consulting or advisory role for AstraZeneca, Gilead Sciences, Merck, and Novartis, and has received a travel grant from Novartis. Margaret Tonda and Mark Shilkrut are employees of and own stocks in Olema Oncology. Nancy U. Lin has received research support paid to her institution from Genentech, Pfizer, Merck, Seattle Genetics, Zion Pharmaceuticals, Olema Oncology, and AstraZeneca. She has received honoraria for consulting work from Puma, Seattle Genetics, Daiichi-Sankyo, AstraZeneca, Denali Therapeutics, Prelude Therapeutics, Olema Oncology, Aleta BioPharma, Affinia Therapeutics, Voyager Therapeutics, Janssen, Blueprint Medicines, Stemline/Menarini, Artera Inc., and Reverie Labs.
Figures



References
-
- American Cancer Society. Breast Cancer Facts and Figures 2022-2024. Atlanta: American Cancer Society, Inc; 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

