Role of mast cell-derived exosomes in exacerbating neuronal injury of experimental cerebral malaria
- PMID: 40598575
- PMCID: PMC12220399
- DOI: 10.1186/s13071-025-06863-3
Role of mast cell-derived exosomes in exacerbating neuronal injury of experimental cerebral malaria
Abstract
Background: Cerebral malaria (CM), a fatal neurological complication of Plasmodium falciparum infection, is partially driven by neuronal injury. Emerging evidence highlights exosomes as vital mediators of mast cell-neuron interactions in neurological disease progression. While mast cells and their exosomes were previously shown to exacerbate experimental cerebral malaria (ECM) severity, the specific role of mast cell-derived exosomes in CM-associated neuronal injury remains unclear.
Methods: Exosomes were isolated from resting and lipopolysaccharide (LPS)-activated P815 mast cells (denoted as RE and AE, respectively) and characterized. These exosomes were administered to ECM mice and Plasmodium berghei ANKA (PbA)-infected red blood cell (iRBC)-stimulated neuronal HT-22 cells to investigate their functional impact and mechanisms.
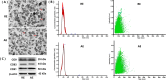
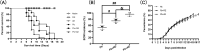
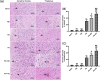
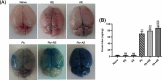
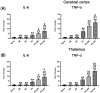
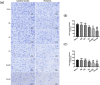
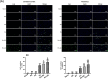
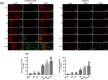
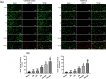
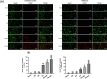
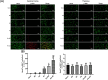
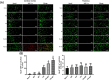
Results: Both RE and AE exhibited spherical morphology (20-100 nm diameter) and expressed exosomal markers (CD9, CD63, and CD81). Compared to infected controls, RE and AE treatments significantly reduced survival time, increased ECM incidence, and exacerbated brain pathology, blood-brain barrier disruption, neuronal injury, and apoptosis. Furthermore, RE and AE administration elevated messenger RNA (mRNA) levels of pro-inflammatory cytokines (interleukin [IL]-6, tumor necrosis factor alpha [TNF-α], and IL-1β) and increased numbers of neurons expressing endoplasmic reticulum (ER) stress markers (GRP78, CHOP, p-IRE1, XBP-1). Notably, AE treatment induced higher morbidity/mortality rates, more severe neuronal injury, and greater ER stress marker expression than RE. In vitro, RE-treated iRBC-stimulated neuronal HT-22 cells showed higher GRP78, CHOP, and XBP-1 mRNA levels than AE-treated cells. MicroRNA (miRNA) sequencing revealed three downregulated miRNAs (miR-330-3p, miR-185-5p, and miR-379-5p) and six upregulated miRNAs (miR-155-5p, miR-423-3p, miR-187-3p, miR-29c-3p, miR-188-5p, miR-192-5p) in AE versus RE, all previously implicated in targeting GRP78, CHOP, or XBP-1.
Conclusions: Mast cell-derived exosomes, particularly those from activated cells (AE), exacerbated ECM neuronal injury through partial activation of ER stress pathways.
Keywords: Cerebral malaria; Endoplasmic reticulum stress; Exosomes; Mast cells; Neuron.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures were reviewed and approved by the Animal Ethics Committee of Guangdong Pharmaceutical University (No. gdpulac2023001). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
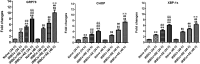
References
-
- WHO. World malaria report 2023. Geneva: World Health Organization; 2023.
-
- Datta D, Conroy A, Castelluccio P, Ssenkusu J, Park G, Opoka R, et al. Elevated cerebrospinal fluid tau protein concentrations on admission are associated with long-term neurologic and cognitive impairment in Ugandan children with cerebral malaria. Clin Infect Dis. 2020;70:1161–8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

