Brain targeted lipid nanoparticles with Hv1 inhibitors alleviate neuroinflammation post-ischemic stroke
- PMID: 40598588
- PMCID: PMC12211482
- DOI: 10.1186/s12951-025-03540-6
Brain targeted lipid nanoparticles with Hv1 inhibitors alleviate neuroinflammation post-ischemic stroke
Abstract
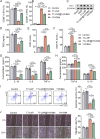
Background: Ischemic stroke (IS) represents a significant global health burden. Current therapeutic options face problems such as window narrowing and reperfusion injury risk. Moreover, with increasing aging and risk factors, novel treatment strategies are urgently needed. NADPH oxidase (NOX)-mediated oxidative stress in microglia is a critical mechanism driving neuroinflammation and cell death. Hv1, a voltage-gated proton channel highly expressed in microglia, synergizes with NOX to generate reactive oxygen species (ROS), exacerbating brain damage. YHV984, a potent Hv1 inhibitor, alleviates post-IS neuroinflammation but faces clinical limitations due to potential toxic side effects and solubility issues. To improve the physicochemical and pharmacokinetic properties of YHV984 for specific Hv1 inhibition in the brain, the multifunctional nanoparticles consisting of a T7-targeting peptide and lipid nanoparticles (LNP) were developed to deliver YHV984 (T7-LNP@YHV984).
Results: The results demonstrated that T7-LNP@YHV984 exhibited good stability and brain targeting capability, effectively crossing the blood-brain barrier (BBB) and accumulating within microglia. This targeted delivery significantly suppressed Hv1 expression and activation of the NLRP3 inflammasome pathway in the damaged brain. Furthermore, it promoted the polarization of microglia towards the M2 phenotype, enhancing the release of anti-inflammatory factors, alleviating neuroinflammation and improved neuronal survival. Additionally, T7-LNP@YHV984 improved survival and facilitated neurological recovery in post-IS mice.
Conclusions: T7-LNP@YHV984 multifunctional nanoparticles with long-term stability emerged as a potent strategy to alleviate reperfusion injury and inhibit neuroinflammation post-IS. By precisely targeting Hv1 in microglia, the nanoparticles effectively suppressed microglia-induced neuroinflammation, minimizing off-target effects. This innovation offers novel insights into stroke treatment and neuroprotective strategies.
Keywords: Brain-targeted LNP; Ischemic stroke; Microglial; Neuroinflammation; Voltage-gated proton channel Hv1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were carried out in accordance with the Institute of Laboratory Animal Resources guidelines. Ethics approval was granted by the Ethics Committee of Southern Medical University. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
- 82001300/National Natural Science Foundation of China
- 82271297/National Natural Science Foundation of China
- 82271297/National Natural Science Foundation of China
- SL2022A04J00214/Guangzhou Science and Technology Project
- GWJJQ2022100104/National Health and Family Planning Commission of the People's Republic of China
LinkOut - more resources
Full Text Sources
Medical

