Host cellular protein RAB33B facilitates influenza viral replication and modulates M2 trafficking by enhancing autophagy
- PMID: 40598642
- PMCID: PMC12219998
- DOI: 10.1186/s13567-025-01560-6
Host cellular protein RAB33B facilitates influenza viral replication and modulates M2 trafficking by enhancing autophagy
Abstract
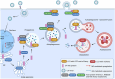
Influenza A virus (IAV) remains a major global health threat. Its M2 protein plays crucial roles in viral fusion, transportation, assembly, and release. Recent studies have shown that IAV impairs host autophagy flux to enhance viral replication. However, the precise mechanisms by which IAV M2 manipulates host cellular autophagy during virus replication remain unclear. In this study, we analysed cellular transcriptional responses of cells to IAV M2 overexpression and identified RAB GTPase protein RAB33B as a key factor. RAB33B was significantly up-regulated by IAV M2 and promoted IAV replication by enhancing autophagy. We further found that autophagy regulates the interaction of IAV M2, RAB33B, and LC3, facilitating M2 membrane trafficking through autophagic-like vesicles. In addition, ATG16L1 (an effector of RAB33B) and TBC1D25 (a GTPase-activating protein for RAB33B) contributed to IAV M2-induced autophagy, thereby affecting viral replication. Collectively, our findings reveal a novel mechanism in which RAB33B is essential for IAV M2 trafficking to the plasma membrane, facilitating viral replication through enhanced autophagy. These insights shed new light on the autophagy-based cellular transport mechanisms of IAV M2 and highlight potential antiviral targets.
Keywords: IAV; M2 protein; RAB33B; autophagy; host–pathogen interaction; membrane trafficking.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare that they have no competing interests.
Figures

Similar articles
-
Influenza A virus induces PI4P production at the endoplasmic reticulum in an ATG16L1-dependent manner to promote the egress of viral ribonucleoproteins.PLoS Biol. 2025 Jul 16;23(7):e3002958. doi: 10.1371/journal.pbio.3002958. eCollection 2025 Jul. PLoS Biol. 2025. PMID: 40668844 Free PMC article.
-
hnRNPM regulates influenza A virus replication through distinct mechanisms in human and avian cells: implications for cross-species transmission.J Virol. 2025 Jun 17;99(6):e0006725. doi: 10.1128/jvi.00067-25. Epub 2025 May 28. J Virol. 2025. PMID: 40434105 Free PMC article.
-
KRT6A Restricts Influenza A Virus Replication by Inhibiting the Nuclear Import and Assembly of Viral Ribonucleoprotein Complex.Viruses. 2025 May 4;17(5):671. doi: 10.3390/v17050671. Viruses. 2025. PMID: 40431683 Free PMC article.
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
-
Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children.Cochrane Database Syst Rev. 2012 Jan 18;1:CD008965. doi: 10.1002/14651858.CD008965.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Apr 10;(4):CD008965. doi: 10.1002/14651858.CD008965.pub4. PMID: 22258996 Updated.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

