Mechanistic insights into direct DNA and RNA strand transfer and dynamic protein exchange of SSB and RPA
- PMID: 40598891
- PMCID: PMC12214019
- DOI: 10.1093/nar/gkaf642
Mechanistic insights into direct DNA and RNA strand transfer and dynamic protein exchange of SSB and RPA
Abstract
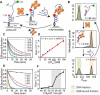
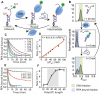
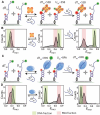
Single-stranded DNA-binding proteins (SSBs) are essential for genome stability, facilitating replication, repair, and recombination by binding single-stranded DNA (ssDNA), recruiting other proteins, and dynamically relocating in response to cellular demands. Using single-molecule fluorescence resonance energy transfer assays, we elucidated the mechanisms underlying direct strand transfer from one locale to another, protein exchange, and RNA interactions at high resolution. Both bacterial SSB and eukaryotic replication protein A (RPA) exhibited direct strand transfer to competing ssDNA, with rates strongly influenced by ssDNA length. Strand transfer proceeded through multiple failed attempts before a successful transfer, forming a ternary intermediate complex with transient interactions, supporting a direct transfer mechanism. Both proteins efficiently exchanged DNA-bound counterparts with freely diffusing molecules, while hetero-protein exchange revealed that SSB and RPA could replace each other on ssDNA, indicating that protein exchange does not require specific protein-protein interactions. Additionally, both proteins bound RNA and underwent strand transfer to competing RNA, with RPA demonstrating faster RNA transfer kinetics. Competitive binding assays confirmed a strong preference for DNA over RNA. These findings provide critical insights into the dynamic behavior of SSB and RPA in nucleic acid interactions, advancing our understanding of their essential roles in genome stability, regulating RNA metabolism, and orchestrating nucleic acid processes.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures





Update of
-
Mechanistic insights into direct DNA and RNA strand transfer and dynamic protein exchange of SSB and RPA.bioRxiv [Preprint]. 2025 Apr 1:2025.04.01.643995. doi: 10.1101/2025.04.01.643995. bioRxiv. 2025. Update in: Nucleic Acids Res. 2025 Jun 20;53(12):gkaf642. doi: 10.1093/nar/gkaf642. PMID: 40236217 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

