The origin of mirror repeats in the human genome
- PMID: 40598895
- PMCID: PMC12214015
- DOI: 10.1093/nar/gkaf619
The origin of mirror repeats in the human genome
Abstract
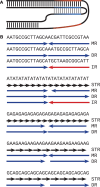
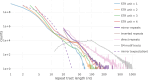
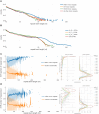
Mirror DNA repeats were found in genomic DNA several decades ago, but their role and the mechanisms leading to their abundance have remained a mystery. The only firmly established functional property was that the subset of long homopurine-homopyrimidine mirror repeats (H-motifs) can form a triple-helical DNA secondary structure (H-DNA). Here, we analyzed the sequence content of mirror repeats in the telomere-to-telomere human genome sequence. Our findings suggest that long mirror repeats in genomic DNA originate exclusively from the expansion of simple tandem repeats (STRs). Strikingly, long H-motifs are highly overrepresented compared to all other mirror repeats and STRs. We hypothesize that long H-motif STRs could be particularly expansion-prone owing to H-DNA-mediated genome instability, pointing to the length at which this structure becomes a significant hindrance.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures


Similar articles
-
Simple sequence repeats and their expansions: role in plant development, environmental response and adaptation.New Phytol. 2025 Jul;247(2):504-517. doi: 10.1111/nph.70173. Epub 2025 May 5. New Phytol. 2025. PMID: 40325839 Free PMC article. Review.
-
Analysis of tandem repeats in seven telomere-to-telomere primate genomes.J Genet. 2025;104:14. J Genet. 2025. PMID: 40539277
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

