Loss of DHX36/G4R1, a G4 resolvase, drives genome instability and regulates innate immune gene expression in cancer cells
- PMID: 40598896
- PMCID: PMC12214017
- DOI: 10.1093/nar/gkaf621
Loss of DHX36/G4R1, a G4 resolvase, drives genome instability and regulates innate immune gene expression in cancer cells
Abstract
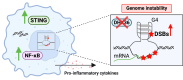
G-quadruplexes (G4s) are four-stranded alternative secondary structures formed by guanine-rich nucleic acids and are prevalent across the human genome. G4s are enzymatically resolved by specialized helicases. Previous in vitro studies showed that DEAH-box helicase 36 (DHX36/G4R1/RHAU) has the highest specificity and affinity for G4 structures. Here, by mapping genome-wide DNA double-strand breaks (DSBs), we demonstrate that knockout of DHX36 helicase increases DSB enrichment at G4 sites and that the presence of the G4 motif is a significant mediator of genome instability at regulatory regions. The loss of DHX36 corresponds with the significant upregulation of NF-κB transcriptional programs, culminating in the production and secretion of proinflammatory cytokines. Loss of DHX36 expression results in the accumulation of cytoplasmic DNA fragments, an increase in the innate immune signaling stimulator of interferon response cGAMP interactor 1 (STING1) expression, and activation of genes involved in immune response pathways. Importantly, higher levels of DHX36 messenger RNA expression in human B-cell acute lymphoblastic leukemia correlate with improved overall survival relative to lower expression of DHX36, highlighting its critical role in preserving genome integrity at a cellular level and in the context of cancer.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures







Update of
-
Loss of DHX36/G4R1, a G4 resolvase, drives genome instability and regulates innate immune gene expression in cancer cells.bioRxiv [Preprint]. 2025 Jan 3:2025.01.03.631217. doi: 10.1101/2025.01.03.631217. bioRxiv. 2025. Update in: Nucleic Acids Res. 2025 Jun 20;53(12):gkaf621. doi: 10.1093/nar/gkaf621. PMID: 39803584 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

