This is a preprint.
Specialized parallel pathways for adaptive control of visual object pursuit
- PMID: 40599160
- PMCID: PMC12212441
- DOI: 10.1101/2025.04.23.650240
Specialized parallel pathways for adaptive control of visual object pursuit
Abstract
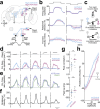
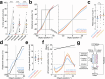
To pursue an unpredictably moving visual object, the brain must generate motor commands that continuously steer the object to the midline of the visual field via feedback. Behavior implies that visual pursuit relies on a feedback loop with flexible gain, but the mechanisms of this "adaptive control" are not well-understood. Here we show that adaptive control in the Drosophila pursuit system involves two parallel feedback loops. One serves to steer the object coarsely toward the midline; the properties of this pathway are relatively constant. The other functions to steer the object precisely to the midline, and its properties are flexible: gain increases when the object is moving away from the midline, when the pursuer is running fast, and during arousal. Genetically suppressing this flexible pathway decreases pursuit performance in aroused males. Our findings show how biological feedback systems can implement adaptive control to drive vigorous error correction while avoiding instability.
Keywords: AOTU019; AOTU025; DNa02; direction selectivity; feedback control; gain scheduling; in vivo electrophysiology; object motion; steering.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Shadmehr R., and Mussa-Ivaldi S. (2012). Biological learning and control (MIT Press; ).
-
- Barnes G.R. (2008). Cognitive processes involved in smooth pursuit eye movements. Brain and Cognition 68, 309–326. - PubMed
-
- Becker W., and Fuchs A.F. (1985). Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp. Brain Res. 57, 562–575. - PubMed
-
- Luebke A.E., and Robinson D.A. (1988). Transition dynamics between pursuit and fixation suggest different systems. Vision Res. 28, 941–946. - PubMed
-
- Schwartz J.D., and Lisberger S.G. (1994). Initial tracking conditions modulate the gain of visuo-motor transmission for smooth pursuit eye movements in monkeys. Vis. Neurosci. 11, 411–424. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
