A multiphase recruitment approach to enroll Latinas diagnosed with cervical cancer into a qualitative study at an academic medical center in the Pacific Northwest
- PMID: 40599171
- PMCID: PMC12209954
- DOI: 10.1017/cts.2025.85
A multiphase recruitment approach to enroll Latinas diagnosed with cervical cancer into a qualitative study at an academic medical center in the Pacific Northwest
Abstract
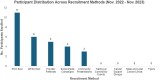
Recruitment of diverse populations into research studies continues to be a challenge. There remains a gap in knowledge and practice on how to best engage with and recruit diverse populations, specifically among Latinos who account for 11% of research participants nationally. Our study focused on Latinas with pre-cervical and cervical cancer in the Pacific Northwest. Our research team took a multilevel approach to diversify recruitment and enrollment processes, focused on methods within healthcare, community-based, and paid media advertisements. This article shares strategies and lessons learned that helped increase participant diversity, meet enrollment goals, and expand relationships with community-based organizations.
Keywords: Latinos; Participant recruitment; diversity; enrollment; oncology.
© The Author(s) 2025.
Conflict of interest statement
The authors declare none.
Figures
Similar articles
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
Interventions targeted at women to encourage the uptake of cervical screening.Cochrane Database Syst Rev. 2021 Sep 6;9(9):CD002834. doi: 10.1002/14651858.CD002834.pub3. Cochrane Database Syst Rev. 2021. PMID: 34694000 Free PMC article.
-
Understanding Barriers and Facilitators to Signing Up for a Mobile-Responsive Registry to Recruit Healthy Volunteers and Members of Underrepresented Communities for Alzheimer's Disease Prevention Studies.J Prev Alzheimers Dis. 2023;10(4):865-874. doi: 10.14283/jpad.2023.67. J Prev Alzheimers Dis. 2023. PMID: 37874109 Free PMC article.
-
Exploring engagement patterns within a mobile health intervention for women at risk of gestational diabetes.Womens Health (Lond). 2025 Jan-Dec;21:17455057251327510. doi: 10.1177/17455057251327510. Epub 2025 Jun 5. Womens Health (Lond). 2025. PMID: 40470610 Free PMC article. Clinical Trial.
-
How to Implement Digital Clinical Consultations in UK Maternity Care: the ARM@DA Realist Review.Health Soc Care Deliv Res. 2025 May;13(22):1-77. doi: 10.3310/WQFV7425. Health Soc Care Deliv Res. 2025. PMID: 40417997 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous