Revolutionizing gastroenterology and hepatology with artificial intelligence: From precision diagnosis to equitable healthcare through interdisciplinary practice
- PMID: 40599184
- PMCID: PMC12207556
- DOI: 10.3748/wjg.v31.i24.108021
Revolutionizing gastroenterology and hepatology with artificial intelligence: From precision diagnosis to equitable healthcare through interdisciplinary practice
Abstract
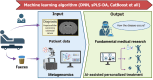
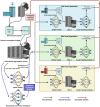
Artificial intelligence (AI) is driving a paradigm shift in gastroenterology and hepatology by delivering cutting-edge tools for disease screening, diagnosis, treatment, and prognostic management. Through deep learning, radiomics, and multimodal data integration, AI has achieved diagnostic parity with expert clinicians in endoscopic image analysis (e.g., early gastric cancer detection, colorectal polyp identification) and non-invasive assessment of liver pathologies (e.g., fibrosis staging, fatty liver typing) while demonstrating utility in personalized care scenarios such as predicting hepatocellular carcinoma recurrence and optimizing inflammatory bowel disease treatment responses. Despite these advancements challenges persist including limited model generalization due to fragmented datasets, algorithmic limitations in rare conditions (e.g., pediatric liver diseases) caused by insufficient training data, and unresolved ethical issues related to bias, accountability, and patient privacy. Mitigation strategies involve constructing standardized multicenter databases, validating AI tools through prospective trials, leveraging federated learning to address data scarcity, and developing interpretable systems (e.g., attention heatmap visualization) to enhance clinical trust. Integrating generative AI, digital twin technologies, and establishing unified ethical/regulatory frameworks will accelerate AI adoption in primary care and foster equitable healthcare access while interdisciplinary collaboration and evidence-based implementation remain critical for realizing AI's potential to redefine precision care for digestive disorders, improve global health outcomes, and reshape healthcare equity.
Keywords: Artificial intelligence; Deep learning; Gastroenterology; Hepatology; Microbiome; Multimodal data integration; Precision medicine.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Revolutionizing surgery: AI and robotics for precision, risk reduction, and innovation.J Robot Surg. 2025 Jan 7;19(1):47. doi: 10.1007/s11701-024-02205-0. J Robot Surg. 2025. PMID: 39776281
-
Scenario-Based Forecasting of ChatGPT's Role as a Research Assistant in Nursing by 2030.Int Nurs Rev. 2025 Sep;72(3):e70055. doi: 10.1111/inr.70055. Int Nurs Rev. 2025. PMID: 40640994
-
Harnessing Artificial Intelligence in Lifestyle Medicine: Opportunities, Challenges, and Future Directions.Cureus. 2025 Jun 8;17(6):e85580. doi: 10.7759/cureus.85580. eCollection 2025 Jun. Cureus. 2025. PMID: 40636636 Free PMC article. Review.
-
The Use of AI for Phenotype-Genotype Mapping.Methods Mol Biol. 2025;2952:369-410. doi: 10.1007/978-1-0716-4690-8_21. Methods Mol Biol. 2025. PMID: 40553344
-
Artificial Intelligence in Cancer Care: Addressing Challenges and Health Equity.Oncology (Williston Park). 2025 Apr 14;39(3):105-110. doi: 10.46883/2025.25921037. Oncology (Williston Park). 2025. PMID: 40266052 Review.
References
-
- Shortliffe EH, Davis R, Axline SG, Buchanan BG, Green CC, Cohen SN. Computer-based consultations in clinical therapeutics: explanation and rule acquisition capabilities of the MYCIN system. Comput Biomed Res. 1975;8:303–320. - PubMed
-
- Listgarten J, Damaraju S, Poulin B, Cook L, Dufour J, Driga A, Mackey J, Wishart D, Greiner R, Zanke B. Predictive models for breast cancer susceptibility from multiple single nucleotide polymorphisms. Clin Cancer Res. 2004;10:2725–2737. - PubMed
-
- Lecun Y, Bottou L, Bengio Y, Haffner P. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278–2324.
-
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. Commun ACM. 2017;60:84–90.
-
- Liu C, Zhao R, Pang M. A fully automatic segmentation algorithm for CT lung images based on random forest. Med Phys. 2020;47:518–529. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

