Ten-Eleven Translocation Family Proteins: Structure, Biological Functions, Diseases, and Targeted Therapy
- PMID: 40599235
- PMCID: PMC12211984
- DOI: 10.1002/mco2.70245
Ten-Eleven Translocation Family Proteins: Structure, Biological Functions, Diseases, and Targeted Therapy
Abstract
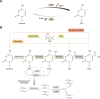
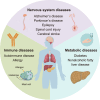
Ten-eleven translocation (TET) family proteins are Fe(II)- and α-ketoglutarate-dependent dioxygenases, comprising three family members: TET1, TET2, and TET3. These enzymes drive DNA demethylation by sequentially oxidizing 5-methylcytosine to 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxylcytosine. Through these reactions, TET proteins remodel the epigenetic landscape and interact with transcription factors and RNA polymerase II to regulate gene expression, cell lineage specification, and embryonic development. Mutations and dysregulation of TETs have been associated with the pathogenesis of various diseases, including the nervous system, immune system, and metabolic diseases, as well as cancers. Therapeutic modulation of TETs may be an effective strategy for the treatment of these diseases. Here, we provide a comprehensive overview of the mechanisms by which TET proteins mediate DNA demethylation and detail their biological functions. Additionally, we highlight recent advances in understanding the molecular mechanisms linking TET dysregulation to disease pathogenesis and explore their potential as therapeutic targets. This review supplements the current understanding of the critical role of epigenetic regulation in disease pathogenesis and further facilitates the rational design of targeted therapeutic agents for diseases associated with mutations and dysregulation of TETs.
Keywords: Biological functions; Demethylation; Diseases; Ten‐eleven translocation; Therapeutic strategies.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
