Development and validation of a clinical sleep assessment tool for patients with cancer during treatment
- PMID: 40599250
- PMCID: PMC12212176
- DOI: 10.1016/j.apjon.2025.100733
Development and validation of a clinical sleep assessment tool for patients with cancer during treatment
Abstract
Objective: Sleep disruption is common among patients with cancer, negatively impacting treatment outcomes, survival, and quality of life. However, it is often overlooked in cancer care. This study aimed to explore shared characteristics of sleep disruption in patients with cancer to facilitate simple and accurate identification in routine clinical practice. A secondary aim was to identify potential biomarkers in urine, serum, or leukocytes associated with sleep disruption before and/or after oncological therapy.
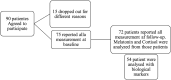
Methods: Ninety cancer patients scheduled for either adjuvant or palliative oncological therapy at Ryhov County Hospital, Jönköping, Sweden, were consecutively enrolled. Of these, 72 completed all questionnaires and provided urine and blood samples at both baseline and three-month follow-up. Data were collected using the 12-item Medical Outcomes Study Sleep Scale (MOS-SS) and the 30-item European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30). Biomarker analysis was conducted on urine and blood samples, and data were analyzed using ordinal factor and Rasch modeling.
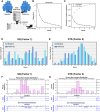
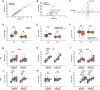
Results: Two distinct factors-Sleep Quality (SQ) and Daytime Sleepiness (DTS)-emerged from the MOS-SS, effectively capturing key aspects of sleep disruption. Both SQ and DTS were strongly associated with sleep-related impairments identified via the EORTC QLQ-C30 and clinical history, but showed no correlation with urinary melatonin or cortisol, serum inflammatory cytokines, or Bmal1 and Per2 gene expression in blood leukocytes. Neither SQ nor DTS was significantly influenced by patient age, body mass index (BMI), or oncological therapy. However, women reported significantly lower DTS compared to men (P < 0.05), while SQ remained unaffected by sex. A simplified scoring tool for SQ and DTS was developed for practical use in clinical oncology settings.
Conclusions: This study identifies SQ and DTS as robust measures of sleep quality and daytime sleepiness in cancer patients. These new factors derived from the MOS-SS can support the early detection and management of sleep disruption in routine oncological care.
Keywords: Cancer; Chemotherapy; Circadian rhythm; Sleep; Sleep disruption.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Charalambous A., et al. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer. 2019;27:2747–2753. - PubMed
-
- Chung K.F., et al. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. 2018;35:365–375. - PubMed
-
- Zengin L., Aylaz R. The effects of sleep hygiene education and reflexology on sleep quality and fatigue in patients receiving chemotherapy. Eur J Cancer Care. 2019;28 - PubMed
-
- Rundo J.V., Downey R. 3rd. Polysomnography. Handb Clin Neurol. 2019;160:381–392. - PubMed
LinkOut - more resources
Full Text Sources

