[Neuroprotective Effects of Anisodine Hydromide in a Rat Model of Vascular Dementia and the Antioxidative Stress Mechanisms Involved]
- PMID: 40599285
- PMCID: PMC12207047
- DOI: 10.12182/20250360505
[Neuroprotective Effects of Anisodine Hydromide in a Rat Model of Vascular Dementia and the Antioxidative Stress Mechanisms Involved]
Abstract
Objective: Vascular dementia (VD) is a common cognitive dysfunction associated with cerebrovascular disease. This study is aimed at investigating the therapeutic effect of anisodine hydromide (AH) on VD and the potential antioxidative stress mechanisms involved.
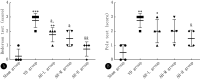
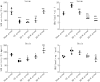
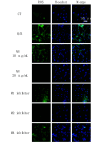
Methods: A VD model was established in Sprague-Dawley (SD) rats through permanent bilateral common carotid artery occlusion. The rats were divided into a sham group, a VD model group, and AH treatment groups receiving AH at low, medium, or high doses (n = 4). The neurological function of the rats in each group was evaluated using the Bederson scale, and limb coordination ability was assessed using the pole climbing test. Superoxide dismutase (SOD) and malondialdehyde (MDA) levels in the serum and brain were measured by enzyme-linked immunosorbent assay (ELISA) to assess the level of oxidative stress. In addition, apoptosis was assessed by TUNEL assay, and reactive oxygen species (ROS) levels in neuronal cells were determined using dichloro-dihydro-fluorescein diacetate (DCFH-DA) probe. The potential mechanism of action of AH on M receptors was investigated using M1-M5 inhibitors.
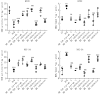
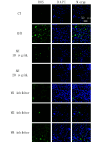
Results: Compared with the sham group, the nerve function and limb coordination of rats in the VD model group were significantly impaired (P < 0.01), and the SOD levels were significantly decreased in the serum ([100.70 ± 18.95] U/mL vs. [44.22 ± 7.11] U/mL, P < 0.001) and the brain ([131.77 ± 8.34] U/mg vs. [84.39 ± 4.10] U/mg, P < 0.01), MDA levels were significantly increased in the serum ([12.03±1.01] nmol/mL vs. [17.74 ± 1.00] nmol/mL, P < 0.001) and the brain ([4.41 ± 0.30] nmol/mg vs. [6.17 ± 0.70] nmol/mg, P < 0.05). AH treatment significantly improved the neurological function and limb coordination ability of VD rats. In comparison with the VD group, the high-dose AH treatment group, in particular, exhibited the most significant increase in SOD levels in the serum ([44.22 ± 7.11] U/mL vs. [98.67 ± 0.86] U/mL, P < 0.001) and the brain ([84.39 ± 4.10] U/mg vs. [162.83 ± 17.36] U/mg, P < 0.001), and the most significant decrease in MDA levels in the serum ([17.74 ± 1.00] nmol/mL vs. [6.68 ± 0.06] nmol/mL, P < 0.001) and the brain ([6.17 ± 0.70] nmol/mg vs. [3.96 ± 0.77] nmol/mg, P < 0.01). AH also reduced the number of TUNEL positive cells (P < 0.01) in a dose-dependent manner. The percentage of apoptotic cells was (36.10 ± 9.07)%, (9.60 ± 5.63)%, and (3.43 ± 0.92)%, respectively, for AH treatment at low, medium, and high concentrations, indicating that AH had an inhibitory effect on apoptosis. According to findings from the in vitro experiments, AH treatment reduced the MDA content (P < 0.01), increased the SOD activity (P < 0.01), and decreased the ROS levels of HT22 and NSC-34 cells in a dose-dependent manner. M2 receptor inhibitors could reduce the ROS level in oxidative stress injury, suggesting that AH, as an M receptor antagonist, might exert its effect by inhibiting the M2 receptor.
Conclusion: AH modulates SOD and MDA levels and reduces oxidative stress injury, thereby improving neurological function and limb coordination and showing potential therapeutic effects in VD. The neuroprotective effects of AH may be related to its antioxidative stress and antiapoptotic mechanisms, and the M2 receptor may be a potential target of its actions. These findings provide an important theoretical basis for the development of new therapeutic strategies for VD.
目的: 血管性痴呆(VD)是一种常见的与脑血管疾病相关的认知功能障碍。本研究旨在探究氢溴酸樟柳碱(AH)对VD的治疗效果及其潜在的抗氧化应激机制。
方法: 通过永久性双侧颈总动脉闭塞法在SD大鼠上建立VD模型,并分为假手术组、VD组和不同剂量的AH治疗组(n=4)。利用Bederson评分法评估各组大鼠的神经功能,并结合爬杆试验分析各组大鼠的肢体协调能力。采用酶联免疫吸附法测定血清和大脑中的超氧化物歧化酶(SOD)和丙二醛(MDA)含量,以评估氧化应激水平。此外,通过TUNEL实验评估细胞凋亡情况,使用DCFH-DA探针检测神经细胞的活性氧(ROS)水平。使用M1-M5抑制剂,探究AH对M受体的可能作用机制。
结果: 与假手术组相比,VD组大鼠神经功能与肢体协调能力受损(P<0.01),血清和大脑中的SOD活性降低〔血清:(100.70±18.95) U/mL vs. (44.22±7.11) U/mL,P<0.001;大脑:(131.77±8.34) U/mg vs. (84.39±4.10) U/mg,P<0.01〕,MDA浓度升高〔血清:(12.03±1.01) nmol//mL vs. (17.74±1.00) nmol//mL,P<0.001;大脑:(4.41±0.30) nmol/mg vs. (6.17±0.70) nmol/mg,P<0.05〕。AH治疗能够改善VD大鼠的神经功能与肢体协调能力,特别是高剂量AH治疗组与VD组相比,SOD活性提高〔血清:(44.22±7.11) U/mL vs. (98.67±0.86) U/mL,P<0.001;大脑:(84.39±4.10) U/mg vs. (162.83±17.36) U/mg,P<0.001〕和MDA浓度降低〔血清:(17.74±1.00) nmol/mL vs. (6.68±0.06) nmol/mL,P<0.001;大脑:(6.17±0.70) nmol/mg vs. (3.96±0.77) nmol/mg,P<0.01〕效果最为显著。AH治疗还减少了TUNEL阳性细胞数量(P<0.01),并呈剂量依赖性,AH浓度从低到高时凋亡细胞占比分别为(36.10±9.07)%、(9.60±5.63)%、(3.43±0.92)%,表明其对细胞凋亡具有抑制作用。在体外实验中,AH处理能够降低HT22和NSC-34细胞的MDA浓度(P<0.01),提高SOD活性(P<0.01),并降低ROS水平,呈现剂量依赖性。M2受体抑制剂可以降低氧化应激损伤中的ROS水平,提示AH作为M受体拮抗剂,可能通过抑制M2受体发挥作用。
结论: 氢溴酸樟柳碱通过调节SOD和MDA水平,减轻氧化应激损伤,改善神经功能和肢体协调能力,对VD具有潜在的治疗效果。AH的神经保护作用可能与其抗氧化应激和抗凋亡机制有关,且M2受体可能是其作用的关键靶点。这些结果为VD治疗新策略开发提供了重要的理论基础。
Keywords: Anisodine; M receptor; Malondialdehyde; Oxidative stress; Superoxide dismutase; Vascular dementia.
© 2025《四川大学学报(医学版)》编辑部 Copyright ©2025 Journal of Sichuan University (Medical Science Edition).
Conflict of interest statement
利益冲突 所有作者均声明不存在利益冲突
Figures

Similar articles
-
Edaravone dexborneol attenuates cognitive impairment in a rat model of vascular dementia by inhibiting hippocampal oxidative stress and inflammatory responses and modulating the NMDA receptor signaling pathway.Brain Res. 2024 Jun 15;1833:148917. doi: 10.1016/j.brainres.2024.148917. Epub 2024 Apr 4. Brain Res. 2024. PMID: 38582415
-
Antioxidant Effects of Moringa oleifera Against Abamectin-Induced Oxidative Stress in the Brain and Erythrocytes of Rats.Chem Biodivers. 2025 May;22(5):e202402709. doi: 10.1002/cbdv.202402709. Epub 2025 Jan 7. Chem Biodivers. 2025. PMID: 39724495
-
Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis.Cochrane Database Syst Rev. 2021 Feb 22;2(2):CD013306. doi: 10.1002/14651858.CD013306.pub2. Cochrane Database Syst Rev. 2021. PMID: 33704781 Free PMC article.
-
A Plant-Based Dietary Supplement Exhibits Significant Effects on Markers of Oxidative Stress, Inflammation, and Immune Response in Subjects Recovering from Respiratory Viral Infection: A Randomized, Double-Blind Clinical Study Using Vitamin C as a Positive Control.Int J Mol Sci. 2025 May 29;26(11):5209. doi: 10.3390/ijms26115209. Int J Mol Sci. 2025. PMID: 40508019 Free PMC article. Clinical Trial.
-
Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972056 Free PMC article.
References
-
- SONG T, SONG X, ZHU C, et al Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer's disease: a meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res Rev. 2021;72:101503. doi: 10.1016/j.arr.2021.101503. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
