Magnesium ions attenuate tendon graft fibrosis during its ligamentization after ACL reconstruction through modulation of fibroblast to myofibroblast trans-differentiation by promoting PGE2 secretion
- PMID: 40599342
- PMCID: PMC12210322
- DOI: 10.1016/j.bioactmat.2025.06.019
Magnesium ions attenuate tendon graft fibrosis during its ligamentization after ACL reconstruction through modulation of fibroblast to myofibroblast trans-differentiation by promoting PGE2 secretion
Abstract
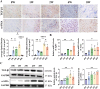
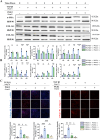
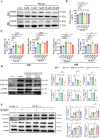
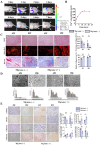
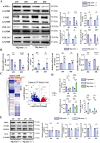
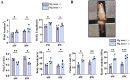
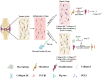
The ligamentization process of the tendon graft in anterior cruciate ligament (ACL) reconstruction is crucial for graft healing quality, thereby affecting knee joint function. Excessive scar tissue, caused by activation of trans-differentiation of fibroblasts to myofibroblasts, rather than orientated collagen fibers with normal composition and structure in the graft mid-substance seriously impacts ligamentization. The elucidation of the underlying mechanism behind the graft fibrosis may facilitate modulation of tendon graft ligamentization. Here, we show that transforming growth factor beta 1 (TGF-β1) was significantly upregulated with ligamentization process, contributing to fibroblast to myofibroblast trans-differentiation and thereby leading to impaired collagen orientation with overproduction of collagen type III. Of note, we verified that prostaglandin E2 (PGE2), a principal mediator of inflammation secreted by macrophages, significantly reversed TGF-β1-induced trans-differentiation of fibroblasts to myofibroblasts. Importantly, magnesium (Mg) ions were found to upregulate PGE2 production in macrophages, ultimately favoring inhibition of scar tissue formation and promoting expression of ligament-like phenotype in the graft mid-substance in rats. Consistently, the rats, with injection of the sodium alginate containing Mg ions into knee joint cavity, exhibited significantly improved gait performance and failure load relative to the control group. These results demonstrate the feasibility of using Mg ions to modulate tendon ligamentization in patients after ACL reconstruction.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Wang J., Xu J., Wang X., Sheng L., Zheng L., Song B., Wu G., Zhang R., Yao H., Zheng N., Yun Ong M.T., Yung P.S., Qin L. Magnesium-pretreated periosteum for promoting bone-tendon healing after anterior cruciate ligament reconstruction. Biomaterials. 2021;268 - PubMed
-
- Font Tellado S., Balmayor E.R., Van Griensven M. Strategies to engineer tendon/ligament-to-bone interface: biomaterials, cells and growth factors. Adv. Drug Deliv. Rev. 2015;94:126–140. - PubMed
LinkOut - more resources
Full Text Sources

