Carrier-Free Nanomedicine Based on Celastrol and Methotrexate for Synergistic Treatment of Breast Cancer via Folate Targeting
- PMID: 40599402
- PMCID: PMC12212079
- DOI: 10.2147/IJN.S516921
Carrier-Free Nanomedicine Based on Celastrol and Methotrexate for Synergistic Treatment of Breast Cancer via Folate Targeting
Abstract
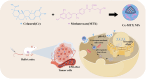
Purpose: To address celastrol(Ce)'s efficacy and toxicity challenges in breast cancer, we first developed a carrier-free, self-targeting nanosystem with synergistic anti-tumor action by leveraging methotrexate (MTX)'s intrinsic folate moiety for active tumor targeting.
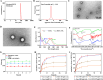
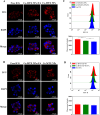
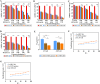
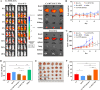
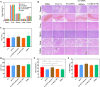
Methods: Ce-MTX nanoparticles (NPs) were prepared using a solvent precipitation method, with formulation parameters optimized. Characterization included particle size, polydispersity index (PDI), encapsulation efficiency (EE), loading efficiency (LE), and TEM. Drug release was investigated under physiological and tumor-mimetic conditions via a dialysis method. Cellular uptake and in vitro anti-tumor effects were evaluated in A549 and 4T1 cell lines. In vivo, tumor distribution and normal tissue accumulation were analyzed in 4T1 tumor-bearing mice. Anti-tumor efficacy and biosafety were evaluated through tumor growth curves, tumor inhibition rates, body weight changes, organ indices, histological analysis, and serum biochemistry.
Results: The optimized Ce-MTX NPs exhibited a particle size of 90.20 nm, PDI of 0.062, and spherical morphology. The EE and LE were 95.15% and 66.53% for Ce, and 95.74% and 33.6% for MTX, respectively. The NPs demonstrated excellent stability over 7 days. Notably, Ce-MTX NPs exhibited pH-dependent drug release, with accelerated release at pH 5.5. Qualitative and quantitative cellular uptake assays revealed significantly higher uptake of Ce-MTX NPs compared to the free drugs, with enhanced folate receptor-targeting in 4T1 cells. Cytotoxicity assays showed stronger anti-tumor activity of Ce-MTX NPs in 4T1 cells compared to the free drug mixture, thus demonstrating the superior synergistic anti-cancer effects achieved by the nanoparticle formulation. Importantly, in vivo studies confirmed substantial tumor growth inhibition and an excellent biosafety profile.
Conclusion: The carrier-free Ce-MTX NPs demonstrated enhanced stability, tumor targeting, and rapid drug release within tumor cells, significantly improving the efficacy and biosafety of breast tumor treatment. These nanoparticles offer a promising strategy for combined cancer therapy and hold great potential for further development in nanomedicine.
Keywords: breast cancer; carrier-free nanoparticles; celastrol; methotrexate; synergistic antitumor efficacy; tumor targeting.
© 2025 Li et al.
Conflict of interest statement
The authors declare no conflicts of interest related to this work.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

