Novel Approach for Evaluating the Effectiveness of Biological Drugs in Patients with Plaque Psoriasis: Real-World Experience of a Single-Centre Study in Poland
- PMID: 40599404
- PMCID: PMC12212070
- DOI: 10.2147/CCID.S517656
Novel Approach for Evaluating the Effectiveness of Biological Drugs in Patients with Plaque Psoriasis: Real-World Experience of a Single-Centre Study in Poland
Abstract
Introduction: Biologic therapies have revolutionized psoriasis treatment, offering targeted interventions that significantly improve outcomes for patients with moderate to severe forms of the disease. In Poland, biologic treatment for psoriasis is funded through the National Health Service's B.47 program, available since 2013.
Aim: This study aimed to perform a retrospective analysis of the effectiveness of biologic treatments during the first year of therapy in patients with plaque psoriasis, treated with biologic agents under the B.47 program at a single center in Poland.
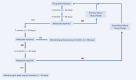
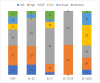
Methods: Medical records of patients with plaque psoriasis who were enrolled in the National Health Service's B.47 drug program at the dermatology department between January 1, 2013, and August 2, 2024, were reviewed. This retrospective chart review utilized secondary data from these records. Ultimately, 159 patients totaling 300 drug-periods were enrolled to the study.
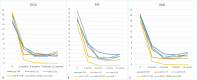
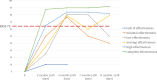
Results: Six distinct patterns of treatment response emerged within the first year. Anti-IL-17AF therapy achieved complete remission (PASI-100) in the shortest time (53.8 days vs 101.7 days for a total population, p<0.05). While treatment effectiveness did not differ between bio-naive and non-bio-naive patients overall, repeated exposures to anti-TNF and anti-IL-12/23 therapies were associated with a diminished clinical response.
Conclusion: The study highlights the critical importance of strategic selection of the treatment to optimize long-term outcomes.
Keywords: PASI; biological drugs; effectiveness; plaque psoriasis; psoriasis severity.
© 2025 Kimak-Pielas et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.Health Technol Assess. 2006 Nov;10(46):1-233, i-iv. doi: 10.3310/hta10460. Health Technol Assess. 2006. PMID: 17083854
-
Guselkumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients: results of the phase III randomized placebo-controlled PROTOSTAR study.Br J Dermatol. 2025 Mar 18;192(4):618-628. doi: 10.1093/bjd/ljae502. Br J Dermatol. 2025. PMID: 39708367 Clinical Trial.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.Health Technol Assess. 2006 Sep;10(31):iii-iv, xiii-xvi, 1-239. doi: 10.3310/hta10310. Health Technol Assess. 2006. PMID: 16948890
References
-
- NHS Drug Programs. Available from: https://www.gov.pl/web/zdrowie/programy-lekowe. Accessed 12, September 2024.
-
- Kimak-Pielas A, Robak E, Zajdel R, Zebrowska A. Demographics, Disease Characteristics, and Treatment Patterns of Patients with Plaque Psoriasis Treated with Biological Drugs: the Experience of a Single-Centre Study in Poland. J Clin Med. 2024;13(24):7647. doi: 10.3390/JCM13247647 - DOI - PMC - PubMed
-
- Mahil SK, Wilson N, Dand N, et al. Psoriasis Treat to Target: defining Outcomes in Psoriasis Using Data from a Real‐world, Population‐based Cohort Study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br J Dermatol. 2020;182:1158–1166. doi: 10.1111/BJD.18333 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

