Distribution and metabolism of iPSC-MSCs in the joint cavity of an osteoarthritis rat model
- PMID: 40599407
- PMCID: PMC12209229
- DOI: 10.3389/fbioe.2025.1555983
Distribution and metabolism of iPSC-MSCs in the joint cavity of an osteoarthritis rat model
Abstract
Introduction: To investigate the metabolism and distribution of iPSC-MSCs in the joint cavity of rats with knee osteoarthritis (KOA).
Methods: The iPSC-MSCs labeled with the Antares2 luciferase gene were injected into the knee joints of rats, and then the metabolism and distribution of the cells in vivo were revealed by imaging and molecular biomarker methods.
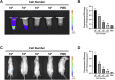
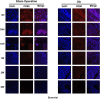
Result: Histopathological results demonstrated that iPSC-MSCs significantly reversed joint tissue damage of arthritic rats. The fluorescence signal of iPSC-MSCs labeled with Antares2 luciferase gene was stable and persistent with high detection sensitivity. The fluorescent signal duration of Antares2-iMSCs in the joint cavity of KOA rats was approximately 2 weeks, which was significantly longer than 1 week in the sham-operated group. The proportion of iPSC-MSCs in the synovial fluid gradually decreased over time, and for the first time, the cells were observed to attach to the synovium first, followed by the meniscus and cartilage.
Discussion: This study was the first to explore the metabolism and distribution of iPSC-MSCs after intra-articular injection by labeling the Antares2 luciferase gene, which provides assurance and theoretical basis for the safety of clinical application of iPSC-MSCs in treating osteoarthritis.
Keywords: Antares2-iMSCs; biodistribution; iPSC-MSCs; metabolism; osteoarthritis.
Copyright © 2025 Yuan, Wang, Du, Zhang, Cheng, Wang, Xu, Yang, Chang, Wei, He and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Human Infrapatellar Fat Pad Mesenchymal Stem Cell-derived Extracellular Vesicles Purified by Anion Exchange Chromatography Suppress Osteoarthritis Progression in a Mouse Model.Clin Orthop Relat Res. 2024 Jul 1;482(7):1246-1262. doi: 10.1097/CORR.0000000000003067. Epub 2024 Apr 19. Clin Orthop Relat Res. 2024. PMID: 38662932 Free PMC article.
-
Multiscale characterization of ultrasmall fluorescent core-shell silica nanoparticles in cartilage and synovial joints reveals rapid cartilage penetration and sustained joint residence.Acta Biomater. 2025 Jun 15;200:313-325. doi: 10.1016/j.actbio.2025.05.031. Epub 2025 May 9. Acta Biomater. 2025. PMID: 40349899
-
Intraarticular corticosteroid for treatment of osteoarthritis of the knee.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD005328. doi: 10.1002/14651858.CD005328. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2006 Apr 19;(2):CD005328. doi: 10.1002/14651858.CD005328.pub2. PMID: 15846755 Updated.
-
Mesenchymal stem cell implantation provides short-term clinical improvement and satisfactory cartilage restoration in patients with knee osteoarthritis but the evidence is limited: a systematic review performed by the early-osteoarthritis group of ESSKA-European knee associates section.Knee Surg Sports Traumatol Arthrosc. 2023 Dec;31(12):5306-5318. doi: 10.1007/s00167-023-07575-w. Epub 2023 Sep 22. Knee Surg Sports Traumatol Arthrosc. 2023. PMID: 37737920 Free PMC article.
-
Intraarticular corticosteroid for treatment of osteoarthritis of the knee.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD005328. doi: 10.1002/14651858.CD005328.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2015 Oct 22;(10):CD005328. doi: 10.1002/14651858.CD005328.pub3. PMID: 16625636 Updated.
References
-
- Chahal J., Gomez-Aristizabal A., Shestopaloff K., Bhatt S., Chaboureau A., Fazio A., et al. (2019). Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl. Med. 8, 746–757. 10.1002/sctm.18-0183 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

