Common Cold Coronavirus Test Positivity Decreased After Widespread SARS-CoV-2 Experience
- PMID: 40599488
- PMCID: PMC12207968
- DOI: 10.1093/ofid/ofaf326
Common Cold Coronavirus Test Positivity Decreased After Widespread SARS-CoV-2 Experience
Abstract
Background: Diverse respiratory viruses, such as the common cold coronaviruses (ccCoVs), respiratory syncytial virus (RSV), and influenza virus (IV), circulated before the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19). We hypothesized that ccCoV, but not RSV or IV, test positivity changed after widespread SARS-CoV-2 infection and COVID-19 vaccination because of shared features among the coronaviruses (CoVs).
Methods: We collected all ccCOVs, RSV, and IV detected at Boston Medical Center from October 2015 to April 2024. We compared virus positivity in the 5 respiratory seasons before the emergence of SARS-CoV-2 in March 2020 with the 2 seasons after the SARS-CoV-2 Omicron variant surge ended around April 2022. We used multivariate linear regression, generalized estimating equations, and multivariate logistic regression analysis to compare total weekly virus detected and test positivity frequency.
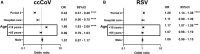
Results: Test positivity for ccCoVs, but not RSV or IV, was significantly lower after widespread SARS-CoV-2 infection and COVID-19 vaccination compared with the seasons before the first documented SARS-CoV-2 case. There was ∼60% lower odds of a ccCoV, but no change in RSV odds, in the seasons after extensive established SARS-CoV-2 immunity from infection and COVID-19 vaccination.
Conclusions: Our results suggest that ccCoV, but not RSV and IV, positivity has decreased significantly in recent respiratory seasons. There are multiple potential mechanisms for the observed ccCoVs decrease, such as cross-reactive immune response among the different but highly related CoVs, along with changing behavioral and health care practices.
Keywords: RSV; SARS-CoV-2; heterotypic immunity; influenza; seasonal coronaviruses.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. The authors have declared that no conflict of interest exists.
Figures


References
-
- McNamara PS, Van Doorn HR. Respiratory viruses and atypical bacteria. Mansons Trop Infect Dis 2014; 215–24.e212.
-
- Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med 2020; 26:1691–3. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

