Implementation of an Online Drug-Drug Interaction Screener for the STRIVE Ensitrelvir Trial for COVID-19
- PMID: 40599489
- PMCID: PMC12207740
- DOI: 10.1093/ofid/ofaf327
Implementation of an Online Drug-Drug Interaction Screener for the STRIVE Ensitrelvir Trial for COVID-19
Abstract
Background: Ensitrelvir is an antiviral agent against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with associated drug-drug interactions (DDIs) through CYP3A, P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and organic anion transporter-3 (OAT-3) inhibition. We present the development and implementation of an online DDI screener to assess interactions during the STRIVE ensitrelvir trial.
Methods: The STRIVE Network is conducting a randomized, double-blind, placebo-controlled trial evaluating ensitrelvir's efficacy and safety in hospitalized adults with coronavirus disease 2019 (COVID-19) and lower respiratory tract involvement. DDI guidance was compiled into a database accessed via a web portal where a multidisciplinary team categorized medications as permitted, prohibited, or conditionally permitted. For prohibited medications, washout periods and start/restart criteria were provided with alternative medication suggestions. Sites could request new medications for addition. After 18 months, a survey was conducted to assess the tool's usefulness.
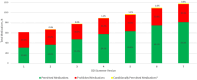
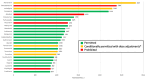
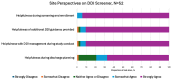
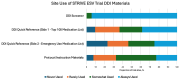
Results: Version 1 of the DDI screener launched in December 2022 with 615 medications, expanding to 1182 through 6 updates by version 7. In 11 cases, prohibited medications were revised to conditionally permit enrollment after dosage adjustments (antihypertensives, anti-infectives, and psychiatric medications). Anticoagulants, immunosuppressants, and emergency use medications posed the greatest challenges due to trial blinding. With 334 participants enrolled across 150 sites in 13 countries, 117192 screener searches were completed by May 2024. The most searched medication classes were antihypertensive, antibiotics, corticosteroids, and anticoagulants. Sites found the DDI screener most helpful during screening/enrollment and valued the washout guidance.
Conclusions: DDI resources for investigational medications like ensitrelvir, with high DDI potential, are crucial for safe conduct of clinical trials. Effective implementation requires a multidisciplinary, iterative approach that incorporates real-time feedback from trial sites.
Keywords: COVID-19; clinical trial; drug–drug interaction; ensitrelvir; online trial resource.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J.P.H. reports research grants paid to his institution, the University of Nebraska Medical Center, and consultancy services to ViiV Healthcare, Inc., and Medscape. S.S. received a research grant from Shionogi. K.C.M. served as a consultant for Shionogi. R.S. is an employee of Shionogi. All other authors have no conflicts of interest.
Figures
References
-
- Centers for Disease Control and Prevention . COVID data tracker. Available at: https://covid.cdc.gov/covid-data-tracker. Accessed May 7, 2024.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

