Rats subject to extracorporeal membrane oxygenation have improved cardiac function following anticoagulation and reversal with factor IXa aptamer-antidote oligonucleotide pair
- PMID: 40599554
- PMCID: PMC12208382
- DOI: 10.1097/CP9.0000000000000122
Rats subject to extracorporeal membrane oxygenation have improved cardiac function following anticoagulation and reversal with factor IXa aptamer-antidote oligonucleotide pair
Abstract
Background and purpose: Unfractionated heparin (UFH) is the most commonly utilized rapid-onset anticoagulant, valued for its potency and reversibility with protamine. However, UFH and protamine are associated with significant side effects, including increased morbidity and mortality, and concerns about sustainability due to the environmental impact of large-scale pig farming for heparin production. This study evaluates an alternative anticoagulant strategy using a factor IXa (FIXa) aptamer paired with a matched oligonucleotide antidote, comparing its efficacy and safety to heparin-protamine in a rat extracorporeal membrane oxygenation (ECMO) model.
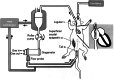
Methods: Twenty-four Sprague-Dawley rats were randomized into two groups: one receiving heparin (600 IU/kg) and protamine (1 mg/100 IU heparin), and the other receiving a cholesterol-modified FIXa aptamer (10 mg/kg) and its antidote (50 mg/kg). Coagulation parameters, platelet counts, inflammatory markers, cardiac function, and histopathology were assessed during and after 60 minutes of ECMO.
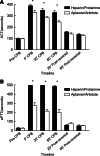
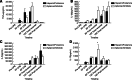
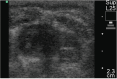
Results: The FIXa aptamer effectively maintained circuit patency without clot formation, comparable to heparin. The antidote rapidly reversed the aptamer's anticoagulant activity, similar to protamine's reversal of heparin. Notably, the aptamer-antidote group demonstrated superior outcomes, including improved mean arterial pressure (58 ± 6 mmHg vs. 54 ± 3 mmHg at 30 minutes; 59 ± 8 mmHg vs. 51 ± 5 mmHg at 3 hours post-ECMO) and cardiac function (shortening fraction: 60 ± 16% vs. 42 ± 8%; P = 0.01). Additionally, the aptamer group exhibited better platelet preservation (platelet count decrease: -288,000 ± 121,000/μL vs. -404,000 ± 89,000/μL; P = 0.03). Inflammatory profiles were similar between groups, except for a transient increase in interleukins 10 (IL-10) in the aptamer group. Histopathological analysis revealed no significant differences in myocardial lesions.
Conclusions: The antidote-controlled anti-FIXa aptamer represents an alternative anticoagulant strategy that may prove useful for managing patients with a history of heparin-induced thrombocytopenia (HIT) and myocardial dysfunction associated with protamine administration.
Keywords: Anticoagulant; Aptamer; Cardiopulmonary bypass; Extracorporeal membrane oxygenation; Heparin.
Copyright © 2025 China Heart House.
Conflict of interest statement
Duke University has submitted patent applications on anticoagulant aptamers and Drs. Shahid M. Nimjee, George A. Pitoc, and Bruce A. Sullenger are inventors on such applications.
Figures

References
-
- Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001;119(1 Suppl):64S–94S. doi:10.1378/chest.119.1_suppl.64s. - PubMed
-
- Izaguirre G, Swanson R, Raja SM, et al. Mechanism by which exosites promote the inhibition of blood coagulation proteases by heparin-activated antithrombin. J Biol Chem 2007;282(46):33609–33622. doi:10.1074/jbc.M702462200. - PubMed
LinkOut - more resources
Full Text Sources
