Substitution of tyrosine with electron-deficient aromatic amino acids improves Ac-PHF6 self-assembly and hydrogelation
- PMID: 40599564
- PMCID: PMC12208052
- DOI: 10.1039/d5ra03251b
Substitution of tyrosine with electron-deficient aromatic amino acids improves Ac-PHF6 self-assembly and hydrogelation
Abstract
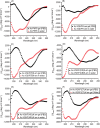
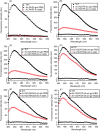
The hexapeptide PHF6 (VQIVYK), an amyloidogenic peptide stretch from human tau, self-assembles via parallel in-register β-sheet formation, wherein Tyr residues are involved in aromatic stacking interactions. Ac-PHF6 (CH3CO-VQIVYK-NH2) forms a viscous solution in water but causes instant gelation of PBS and cell culture media. Aromatic substitutions have been reported in the literature to modulate the self-assembly of peptides. In this study, we perturbed the electronic properties of the sole aromatic residue in Ac-PHF6 and studied hydrogelation. The Tyr residue was substituted with Phe, and the phenyl moiety was then substituted with various electron-withdrawing groups at the para position. All peptides caused PBS gelation with comparable rheological properties. The structures underlying the hydrogels were β-sheet fibrils. The electron-deficient aromatic moieties improved self-assembly and hydrogelation. Ac-PHF6 and no other aromatic analog except the one having p-(trifluoromethyl)phenylalanine caused the gelation of deionized water. Water gelation caused by the p-(trifluoromethyl)phenylalanine-containing analog is likely hydrophobicity-driven.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures






Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Exercise versus airway clearance techniques for people with cystic fibrosis.Cochrane Database Syst Rev. 2022 Jun 22;6(6):CD013285. doi: 10.1002/14651858.CD013285.pub2. Cochrane Database Syst Rev. 2022. PMID: 35731672 Free PMC article.
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
-
Pharmacotherapies for sleep disturbances in dementia.Cochrane Database Syst Rev. 2016 Nov 16;11(11):CD009178. doi: 10.1002/14651858.CD009178.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Nov 15;11:CD009178. doi: 10.1002/14651858.CD009178.pub4. PMID: 27851868 Free PMC article. Updated.
References
LinkOut - more resources
Full Text Sources

