An update on recent advances in fluorescent materials for fluorescence molecular imaging: a review
- PMID: 40599579
- PMCID: PMC12208293
- DOI: 10.1039/d5ra03102h
An update on recent advances in fluorescent materials for fluorescence molecular imaging: a review
Abstract
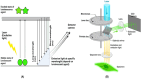
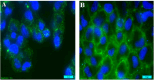
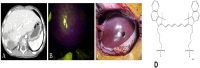
Fluorescence molecular imaging (FMI) is a powerful imaging technique used primarily in biomedical research and clinical applications to visualize molecular and cellular processes of tumors and other diseases. FMI involves the use of fluorescent molecules (fluorophores) that absorb light at one wavelength and emit it at a longer wavelength. These fluorophores can be attached to specific molecules and markers (such as proteins, nucleic acids, or small molecules) in a biological sample. FMI typically offers non-radioactive and safe, real-time and higher spatial resolution compared to positron emission tomography (PET) for superficial tumors. Additionally, sensitivity and specificity of FMI for superficial tumors in better than PET is some cases. However, FMI and the materials used in molecular imaging (MI) have revolutionized biomedical research, diagnostics, and therapeutic monitoring. In contrast, despite their significant contributions, several challenges remain to be solved to improve the effective application of fluorescence-based techniques. These challenges are related to poor tissue penetration depth, background autofluorescence, photobleaching of fluorophores, low signal-to-noise ratio in deep tissues and the necessity for biocompatible and photostable probes. Hence, ongoing improvements in probe development, imaging technologies and analytical methods are required to overcome current challenges. Future advancements in fluorescence materials and imaging techniques hold promise for making MI more accurate, efficient and applicable for clinical and research scenarios. This review gives an overview of recent advances in the materials used in MI and findings of FMI. Finally, limitations of FMI are highlighted and recommendations for future research directions are proposed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors do not have conflicts of interest to disclose.
Figures


Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
References
-
- Oprea-Lager D.-E. MacLennan S. Bjartell A. Briganti A. Burger I. A. de Jong I. De Santis M. Eberlein U. Emmett L. Fizazi K. Gillessen S. Herrmann K. Heskamp S. Iagaru A. Jereczek-Fossa B. A. Kunikowska J. Lam M. Nanni C. O'Sullivan J. M. Panebianco V. Sala E. Sathekge M. Sosnowski R. Tilki D. Tombal B. Treglia G. Tunariu N. Walz J. Yakar D. Dierckx R. Sartor O. Fanti S. Eur. Urol. 2024;85:49–60. - PubMed
-
- Guja K. E. Behr G. Bedmutha A. Kuhn M. Nadel H. R. Pandit-Taskar N. Semin. Nucl. Med. 2024;54:438–455. - PubMed
-
- Shimomura O. Johnson F. H. Saiga Y. J. Cell. Comp. Physiol. 1962;59:223–239. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

