Assessing time requirements of two models of SARS-CoV-2 screening and testing in routine healthcare services in Kenya and Cameroon: a descriptive study
- PMID: 40599610
- PMCID: PMC12211281
- DOI: 10.1136/bmjph-2024-001154
Assessing time requirements of two models of SARS-CoV-2 screening and testing in routine healthcare services in Kenya and Cameroon: a descriptive study
Abstract
Introduction: Incorporating SARS-CoV-2 antigen-detecting rapid diagnostic tests (Ag-RDTs) into routine care settings can facilitate efficient case identification and management in low-resource settings. We assessed the time required to complete SARS-CoV-2 screening and Ag-RDT testing in maternal, neonatal and child health (MNCH), HIV and tuberculosis clinics in selected facilities in Kenya and Cameroon.
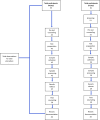
Methods: We conducted a descriptive, time-motion analysis comparing SARS-CoV-2 screening and testing through standard-of-care 'screen-and-test' (ST) and 'test-all' (TA) models. Study staff observed and documented time in minutes taken by healthcare workers to provide SARS-CoV-2 services. Time taken per model was compared using the Wilcoxon rank-sum (Mann-Whitney) or Kruskal-Wallis test.
Results: A total of 116 observations of SARS-CoV-2 screening and testing using Ag-RDTs were conducted. The overall time spent on SARS-CoV-2 activities for clients was a median of 34 min (IQR: 25, 41) for ST sites and 21 min (IQR: 15, 27) at TA sites, p=0.001. Screening took a median time of 3 min (IQR: 2, 7) at ST sites. Among activities observed, test processing took the longest at 19 min (IQR: 17, 21) in ST sites versus 16 min (IQR: 15, 18.5) in TA sites, p=0.001.
Conclusions: SARS-CoV-2 screening and testing services in routine healthcare services took slightly longer in the ST model compared with the TA model, with the majority of additional time needed for sample processing/testing in both models. However, in high-volume clinics, the additional 21 min of personnel and client time needed to test every attendee may not be feasible compared with the 34 min of additional time needed for testing only eligible attendees. When considering the model to use, clinic workload and human resource availability need to be considered to manage the time required for providing SARS-CoV-2 services.
Trial registration number: NCT05382130 17 May 2022.
Keywords: COVID-19; Public Health; SARS-CoV-2.
Copyright © Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. Published by BMJ Group.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Workplace interventions to reduce the risk of SARS-CoV-2 infection outside of healthcare settings.Cochrane Database Syst Rev. 2022 May 6;5(5):CD015112. doi: 10.1002/14651858.CD015112.pub2. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Apr 10;4:CD015112. doi: 10.1002/14651858.CD015112.pub3. PMID: 35514111 Free PMC article. Updated.
References
-
- Duma Z, Chuturgoon AA, Ramsuran V, et al. The challenges of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing in low-middle income countries and possible cost-effective measures in resource-limited settings. Global Health. 2022;18:5. doi: 10.1186/s12992-022-00796-7. - DOI - PMC - PubMed
-
- Karanja S, Aduda J, Thuo R, et al. Utilization of digital tools to enhance COVID-19 and tuberculosis testing and linkage to care: A cross-sectional evaluation study among Bodaboda motorbike riders in the Nairobi Metropolis, Kenya. PLoS One. 2023;18:e0290575. doi: 10.1371/journal.pone.0290575. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous