Treatment of bacterial biothreat agents with a novel purified bioactive lactoferrin affects both growth and biofilm formation
- PMID: 40599649
- PMCID: PMC12209266
- DOI: 10.3389/fcimb.2025.1603689
Treatment of bacterial biothreat agents with a novel purified bioactive lactoferrin affects both growth and biofilm formation
Abstract
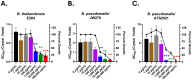
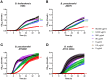
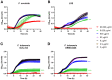
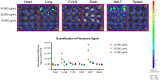
Lactoferrin is known to exhibit broad spectrum activity against a multitude of bacteria, fungi, and viruses due to its multi-functional mode of action. Recently, Lactea Therapeutics and its affiliates have developed a novel, patent-pending technology to purify naturally derived bovine lactoferrin (Lactea Lf) for use as a medical countermeasure that was not previously available. To assess the efficacy of Lactea Lf against biothreat pathogens, we performed biofilm inhibition assays and generated dose-response curves against Burkholderia pseudomallei, Burkholderia mallei, and Francisella tularensis for proof-of-principle studies. Here, we show that Lactea Lf can significantly inhibit biofilm and decrease the overall growth in a dose dependent manner for all Burkholderia species tested. Of note, Lactea Lf was found to completely inhibit biofilm formation by virulent B. pseudomallei without observing complete growth inhibition. The growth of F. tularensis was also significantly inhibited when cultured in the presence of Lactea Lf and appeared more sensitive to treatment when compared to B. pseudomallei. Based on these results, a pneumonic infection model using the F. tularensis LVS strain was performed prophylactically administering Lactea Lf and continuing treatment post challenge. No protection was observed in this model which prompted biodistribution studies using fluorescent tagged Lactea Lf. These experiments revealed that therapeutic material was mainly confined to the NALT region following intranasal delivery and then quickly dispersed or inactivated suggesting that future formulation and delivery method could be addressed to increase in vivo treatment efficacy. Taken together, these data support that Lactea Lf is a potentially new candidate for further studies as a broad-spectrum antimicrobial medical countermeasure with efficacy against several high priority biodefense-related bacterial pathogens.
Keywords: Burkholderia; Francisella; biofilm; glanders; lactoferrin; melioidosis; tularemia.
Copyright © 2025 Xander, Martinez, Toothman, Gardner, Qiu, Snedeker, Bender, Hlubb, Burke, Bozue and Mlynek.
Conflict of interest statement
All data in this paper was produced by USAMRIID. Lactea Therapeutics LT, a for-profit company, is affiliated with three authors on this paper Authors JS, MB, CH. Members of LT did not produce any data used in this paper which was performed fully independently, but supplied material for testing without cost, input on experimental design, and feedback on language in this publication. Author JS is an employee of LT. Authors MB and CH are employed through LT’s parent company, Agrilogics Group AG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Aguila A., Herrera A. G., Morrison D., Cosgrove B., Perojo A., Montesinos I., et al. (2001). Bacteriostatic activity of human lactoferrin against Staphylococcus aureus is a function of its iron-binding properties and is not influenced by antibiotic resistance. FEMS Immunol. Med. Microbiol. 31, 145–152. doi: 10.1111/j.1574-695X.2001.tb00511.x - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

