Therapeutic interventions aimed at cccDNA: unveiling mechanisms and evaluating the potency of natural products
- PMID: 40599653
- PMCID: PMC12209257
- DOI: 10.3389/fcimb.2025.1598872
Therapeutic interventions aimed at cccDNA: unveiling mechanisms and evaluating the potency of natural products
Abstract
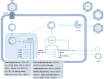
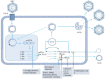
Hepatitis B virus (HBV) infection persists as a formidable global health predicament, imposing a substantial burden on public health. It not only elevates the risk of cirrhosis but also significantly heightens the incidence of hepatocellular carcinoma (HCC), thereby exacerbating the complexity of managing this disease. Central to the intractability of chronic hepatitis B is the tenacious persistence of covalently closed circular DNA (cccDNA) within the nuclei of infected hepatocytes. This cccDNA serves as a stable transcriptional template, continuously fueling the production of viral components and rendering the virus refractory to current antiviral interventions. The attainment of a definitive cure for HBV infection hinges upon the development of innovative antiviral strategies that can precisely and effectively target and eliminate cccDNA from the infected liver cells. In this regard, natural products have emerged as a promising source of potential therapeutics. This comprehensive review delves into the natural products that have shown promise in specifically targeting cccDNA. It meticulously elucidates the intricate molecular mechanisms through which these natural compounds modulate cccDNA activity, such as interfering with cccDNA formation, disrupting its epigenetic regulation, or inhibiting its transcriptional output. Developing innovative strategies to target and eliminate cccDNA is crucial for curing HBV infection, and natural products hold great promise. This review details several natural products with cccDNA-targeting potential, supported by clear mechanisms and data. Dehydrocheilanthifolin (DHCH) from Corydalis saxicola inhibits HBsAg and HBeAg secretion in HepG2.2.15 cells. It may disrupt viral processes like pgRNA packaging or DNA polymerase activity, with IC50 values for reducing extracellular, intracellular DNA, and cccDNA at 15.08 μM, 7.62 μM, and 8.25 μM respectively. Methyl helicterate from Helicteres angustifolia decreases HBsAg, HBeAg, HBV DNA, and cccDNA in HepG2.2.15 cells. 15.8 μM reduces intracellular cccDNA. Curcumin from turmeric reduces viral load and cccDNA in d-imHCs; 30µM halves cccDNA levels. Epigallocatechin gallate (EGCG) from green tea hinders viral transcription and replication. 22.9μg/ml EGCG lowers cccDNA by about 60%. Asiaticoside from Hydrocotyle sibthorpioides inhibits HBsAg, HBeAg, and cccDNA in HepG2.2.15 cells. Notably, despite extensive research, no natural product has yet obtained clinical validation for cccDNA clearance, highlighting the significant translational gap between pre-clinical research and clinical application. By elucidating these molecular mechanisms, this review aims to contribute to the development of HBV-targeted therapies, offering valuable insights for designing novel therapeutic agents and optimizing existing treatment regimens, ultimately advancing the quest for an effective cure for HBV infection.
Keywords: HBV; cccDNA; covalently closed circular DNA; hepatitis B virus; natural product.
Copyright © 2025 Hao, Li and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

