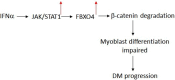
IFNα/JAK/STAT1 Axis-Induced FBXO4 Modulates Muscle Cell Differentiation via β-Catenin Degradation in Dermatomyositis
- PMID: 40599691
- PMCID: PMC12212093
- DOI: 10.2147/JIR.S506056
IFNα/JAK/STAT1 Axis-Induced FBXO4 Modulates Muscle Cell Differentiation via β-Catenin Degradation in Dermatomyositis
Abstract
Purpose: Dermatomyositis (DM) is an inflammatory myopathy characterized by chronic muscle inflammation and damage. Although the pathogenesis of DM has been widely reported to be related to chronic inflammation, the role of ubiquitin E3 ligases in DM remains unclear. In the current study, we aimed to investigate the biological roles of ubiquitin E3 ligase in DM.
Methods: Deseq2 was used to screen the differential express genes in DM public datasets. Quantitative real time PCR and Western blot were used to examine the mRNA and protein levels. Co-immunoprecipitation assays were used to investigate the protein interactions between proteins. Dual-luciferase reporter assays were applied to investigate the regulation between transcription factors and targets.
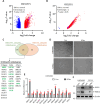
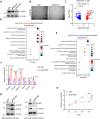
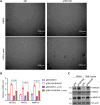
Results: In the current study, we screened public DM-related datasets and focused on ubiquitin-proteasome-related enzymes. Ultimately, we identified the ubiquitin E3 ligase FBXO4. FBXO4 was significantly upregulated in DM muscle tissues compared to normal controls. In human muscle cells (LHCN-M2), FBXO4 knockout led to significant upregulation of genes related to muscle cell differentiation and significant downregulation of genes enriched in cell cycle pathways, as revealed by RNA-seq. These results suggest that FBXO4 knockout promotes muscle cell differentiation. Mechanistic studies showed that FBXO4 ubiquitinates and degrades β-catenin, thereby inhibiting the Wnt/β-catenin signaling pathway and suppressing muscle cell differentiation. On the other hand, FBXO4 may promote muscle cell apoptosis in DM by degrading MCL1. Additionally, we found that FBXO4 is regulated by the IFNα/JAK/STAT1 signaling pathway in DM and identified FBXO4 as a direct target of STAT1.
Conclusion: In conclusion, our findings suggest that IFNα/JAK/STAT1 signaling pathway elevates the expression of FBXO4 in DM and then it contributes to muscle atrophy by inhibiting differentiation and promoting apoptosis. Targeting FBXO4 may offer a novel therapeutic approach for DM.
Keywords: FBXO4; IFNα/JAK/STAT1 axis; Wnt/β-catenin signaling; dermatomyositis; muscle cell differentiation.
© 2025 Yin et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures


Similar articles
-
A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies.J Am Acad Dermatol. 2006 Apr;54(4):597-613. doi: 10.1016/j.jaad.2005.10.041. Epub 2006 Jan 23. J Am Acad Dermatol. 2006. PMID: 16546580
-
Umbelliferone attenuates diabetic sarcopenia by modulating mitochondrial quality and the ubiquitin-proteasome system.Phytomedicine. 2025 Aug;144:156930. doi: 10.1016/j.phymed.2025.156930. Epub 2025 May 31. Phytomedicine. 2025. PMID: 40483791
-
Use of Janus kinase inhibitors in dermatomyositis: a systematic literature review.Clin Exp Rheumatol. 2023 Mar;41(2):348-358. doi: 10.55563/clinexprheumatol/hxin6o. Epub 2022 Jun 28. Clin Exp Rheumatol. 2023. PMID: 35766013 Free PMC article.
-
Caveolin-1 inhibits the proliferation and invasion of lung adenocarcinoma via EGFR degradation.Sci Rep. 2025 Jul 1;15(1):21654. doi: 10.1038/s41598-025-05259-8. Sci Rep. 2025. PMID: 40594106 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

