Residual DNA impurities in AAV vectors-nature and transcription
- PMID: 40599703
- PMCID: PMC12209921
- DOI: 10.1016/j.omtm.2025.101503
Residual DNA impurities in AAV vectors-nature and transcription
Abstract
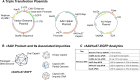
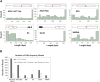
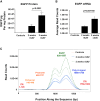
Recombinant adeno-associated viruses (rAAVs) produced by transfecting DNA plasmids into mammalian cells can inadvertently package host cell DNA (hcDNA) and plasmid DNA inside their capsids. Although the percentage of these DNA impurities is low compared to the rAAV genome in vector preparations, it is essential to characterize the DNA impurities in gene therapy products due to the theoretical risks associated with unwanted gene expression and potential immunogenicity and oncogenicity in treated patients. We performed long-read sequencing in rAAV vector, with a focus on analyzing residual, non-transgene DNA within the capsids. Although we detected host cell and residual plasmid DNA impurities, they were predominantly incomplete sequences without coding potential. This indicated that while DNA impurities may be present in rAAV preparations, host cell and residual plasmid genes were unlikely to be expressed. This was supported by RNA sequencing (RNA-seq) analyses that showed minimal plasmid RNA transcripts and host cell RNA transcripts in the livers of mice dosed with rAAV. Overall, the results from these studies enable data-based risk assessment of co-packaged DNA impurities and a better understanding of potential adverse effects associated with rAAV gene therapy.
Keywords: AAV product quality assessment; AAV safety assessment; DNA impurities; PacBio sequencing; gene therapy; host cell DNA transcription; rAAV; residual DNA.
© 2025 The Author(s).
Conflict of interest statement
This work was supported by Ultragenyx Pharmaceutical Inc.
Figures

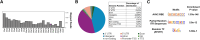

References
-
- Approved Cellular and Gene Therapy Products. 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-produ...
-
- Clinical Trials - Gene Therapy. https://clinicaltrials.gov/search?term=%22gene%20therapy%22
LinkOut - more resources
Full Text Sources
Other Literature Sources

