Efficacy and safety of tranexamic acid administration for subarachnoid hemorrhage: a systematic review and meta-analysis
- PMID: 40599738
- PMCID: PMC12209260
- DOI: 10.3389/fneur.2025.1617817
Efficacy and safety of tranexamic acid administration for subarachnoid hemorrhage: a systematic review and meta-analysis
Abstract
Introduction: Aneurysmal subarachnoid hemorrhage (SAH) carries a high risk of early rebleeding and worsens prognosis. Tranexamic acid (TXA), an antifibrinolytic agent, can prevent rebleeding; however, its effects on mortality and neurological outcomes remain controversial.
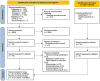
Methods: This review evaluated the efficacy and safety of TXA with SAH. MEDLINE, CENTRAL, EMBASE, ICTRP, and ClinicalTrials.gov were systematically searched for randomized controlled trials (RCTs) and non-randomized studies of interventions (NRSIs) to assess TXA use in SAH. Studies comparing TXA with controls with SAH were included. The primary outcome was the mortality; secondary outcomes included neurological outcomes, rebleeding, thromboembolism, delayed cerebral ischemia (DCI), hydrocephalus, and adverse events. The certainty of evidence was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach.
Results: Fifteen RCTs (3,109 patients) and nine NRSIs (1,506 patients) were included. RCTs demonstrated that TXA likely does not reduce mortality (risk ratio [RR], 1.00; 95% confidence interval [CI], 0.82-1.22; moderate certainty) and neurological outcome, and may not increase thromboembolism and DCI. However, TXA probably reduces rebleeding but probably increases hydrocephalus. The NRSIs results were similar.
Discussion: Although routine use is not supported, TXA may be considered for high-risk patients when early aneurysm treatment is unavailable.
Systematic review registration: https://osf.io/yp78b/.
Keywords: meta-analysis; rebleeding; subarachnoid hemorrhage; systematic review; tranexamic acid.
Copyright © 2025 Imai, Ito, Okano, Inoue, Terayama, Okamoto, Hifumi, Fujimoto, Fujiwara and Kuroda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Effect of tranexamic acid on rebleeding, mortality, and hydrocephalus in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis.J Clin Neurosci. 2025 May;135:111189. doi: 10.1016/j.jocn.2025.111189. Epub 2025 Mar 20. J Clin Neurosci. 2025. PMID: 40117766
-
Antifibrinolytic drugs for treating primary postpartum haemorrhage.Cochrane Database Syst Rev. 2018 Feb 20;2(2):CD012964. doi: 10.1002/14651858.CD012964. Cochrane Database Syst Rev. 2018. PMID: 29462500 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage.Cochrane Database Syst Rev. 2022 Nov 9;11(11):CD001245. doi: 10.1002/14651858.CD001245.pub3. Cochrane Database Syst Rev. 2022. PMID: 36350005 Free PMC article.
-
Tranexamic acid for percutaneous nephrolithotomy.Cochrane Database Syst Rev. 2023 Oct 26;10(10):CD015122. doi: 10.1002/14651858.CD015122.pub2. Cochrane Database Syst Rev. 2023. PMID: 37882229 Free PMC article.
References
-
- Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and Meta-analysis. JAMA Neurol. (2019) 76:588–97. doi: 10.1001/jamaneurol.2019.0006, PMID: - DOI - PMC - PubMed
-
- Chandra B. Treatment of subarachnoid haemorrhage from ruptured intracranial aneurysm with tranexamic acid: a double-blind clinical trial. Ann Neurol. (1978) 3:502–4. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

