The clinical features, muscle pathology, and role of autophagy in anti-Ku-positive patients
- PMID: 40599787
- PMCID: PMC12209196
- DOI: 10.3389/fimmu.2025.1608735
The clinical features, muscle pathology, and role of autophagy in anti-Ku-positive patients
Abstract
Aims: This study aimed to examine the clinical and muscle histological characteristics of anti-Ku-positive patients. A preliminary investigation into the involvement of autophagy was conducted as well.
Methods: Clinical characteristics, laboratory findings, and muscle histological features were collected from patients with isolated anti-Ku antibodies at the Department of Neurology, First Medical Center of the PLA General Hospital, between February 2011 to June 2024. Autophagy-related protein levels were semi-quantitatively assessed on muscle tissue samples using western blot (WB), with sporadic inclusion body myositis (sIBM) and immune-mediated necrotizing myopathy (IMNM) patients as comparison groups.
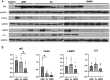
Results: A total of 6 patients were recruited in the study (50% female, mean age at onset 47.6 ± 15.56 years, mean disease duration 7 ± 5.58 months). Extramuscular involvement was observed in most cases, including subcutaneous edema (33.3%), skin rash (33.3%), hyperpigmentation (33.3%), hair loss (33.3%), arthralgia (50%), and interstitial lung disease (ILD) (33.3%), etc. Coexisting connective tissue diseases included systemic sclerosis (SSc) (83.3%), systemic lupus erythematosus (SLE) (16.7%), and arthritis (16.7%). The distribution of muscle weakness was generally symmetrical and proximal (83.3%). Distal (50%) and axial (50%) muscle weakness could also be found. 2 patients exhibited peripheral nerve damage and myogenic damage in EMG, while 4 showed myogenic damage. Creatine kinase (CK) was mildly or moderately elevated. Muscle biopsy demonstrated two patterns: a neurogenic atrophy pattern and a myositis pattern characterized by a varying degree of necrotizing fibers (100%) with rimmed vacuoles (50%) or non-rimmed vacuoles (50%). Immunohistochemical (IHC) analysis revealed sarcolemma deposition of major histocompatibility complex class I (MHC-I) (83.3%) and MHC-II (83.3%), as well as predominant CD68-positive inflammatory infiltrates (66.7%). IHC for p62 revealed a sarcoplasmic punctate pattern (50%), along with a focal coarse staining pattern (50%) and occasional fine granular staining (33.3%). Electron microscopy (EM) demonstrated filamentous and lipid accumulation within vacuoles. WB analysis showed that p62 levels significantly differed between the anti-Ku and IMNM groups. Additionally, Parkin levels were highest in sIBM, while lysosome-associated membrane protein 2 (LAMP2) and microtubule-associated protein 1A/1B-light chain 3 (LC3) expression was highest in the anti-Ku-positive group in tendency.
Conclusion: The muscular features were heterogeneous in anti-Ku-positive patients. A predominant myositis pattern was characterized by necrotizing fibers and vacuolar changes in muscle histology, which differ from sIBM and IMNM. Autophagy appeared to be a key mechanism implicated in the pathogenesis.
Keywords: anti-Ku antibody; autoimmune disease; autophagy; myositis; skeletal muscle pathology.
Copyright © 2025 Qiao, Lin, Liu, Liu, Li, Chen and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

