Pulmonary delivery of excipient-free tobramycin DPIs for the treatment of Pseudomonas aeruginosa lung infection with CF
- PMID: 40599797
- PMCID: PMC12208834
- DOI: 10.3389/fphar.2025.1528905
Pulmonary delivery of excipient-free tobramycin DPIs for the treatment of Pseudomonas aeruginosa lung infection with CF
Abstract
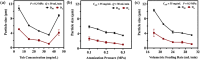
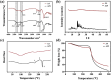
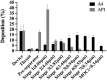
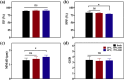
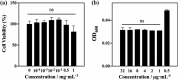
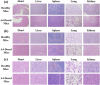
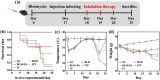
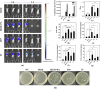
Pseudomonas aeruginosa infection has become a widespread problem in patients with cystic fibrosis (CF). A safe and effective manufacturing method is required to produce antibiotic dry powder inhalations (DPIs) which can be effectively delivered to treat lung infections. In this study, an excipient-free tobramycin inhalable powder was prepared using spray freeze-drying (SFD) method. The mass median aerodynamic diameters (MMAD) of optimized inhalable powder prepared by SFD was 1.30 µm, and the fine particle fractions (FPF) reached 83.31%. In both in vitro and in vivo safety and activity studies, the inhalable powder showed excellent safety performance at both animal and cellular levels, with a minimum inhibitory concentration (MIC) of 0.5 μg/mL. Compared with intravenous injection, inhalation of excipient-free tobramycin inhalable powder had a better effect in the infected mouse model because of its amorphous state. This study demonstrates that excipient-free tobramycin inhalable powder with good delivery and deposition performance can be successfully obtained using the SFD method. Inhalation of excipient-free tobramycin inhalable powder has the potential to be a promising strategy for treating pulmonary infections caused by P. aeruginosa in patients with CF.
Keywords: Pseudomonas aeruginosa infection; dry powder inhalations (DPIs); pulmonary drug delivery; spray freeze-drying (SFD); tobramycin.
Copyright © 2025 Cheng, Huang, Zhang, Chen, Zhang, Lin, Yang, Kanye, Zhang, Xue, Shi, Dong and Li.
Conflict of interest statement
Authors QZ and KS were employed by Suzhou Inhal Pharma Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
Preparation and in vitro characterization of inhalable cannabidiol dry powder for treating chronic obstructive pulmonary disease.Int J Pharm. 2025 Sep 15;682:125892. doi: 10.1016/j.ijpharm.2025.125892. Epub 2025 Jun 25. Int J Pharm. 2025. PMID: 40578460
-
Advanced Spray-Dried Inhalable Microparticles/Nanoparticles of an Innovative Mitophagy Activator for Targeted Lung Delivery: Design, Comprehensive Characterization, Human Lung Cell Culture, and In Vitro Aerosol Dispersion Performance.ACS Pharmacol Transl Sci. 2024 Oct 15;7(11):3540-3558. doi: 10.1021/acsptsci.4c00436. eCollection 2024 Nov 8. ACS Pharmacol Transl Sci. 2024. PMID: 39539257
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
-
Inhaled anti-pseudomonal antibiotics for long-term therapy in cystic fibrosis.Cochrane Database Syst Rev. 2022 Nov 14;11(11):CD001021. doi: 10.1002/14651858.CD001021.pub4. Cochrane Database Syst Rev. 2022. PMID: 36373968 Free PMC article.
References
-
- Alhajj N., O’Reilly N. J., Cathcart H. (2021). Designing enhanced spray dried particles for inhalation: a review of the impact of excipients and processing parameters on particle properties. Powder Technol. 384, 313–331. 10.1016/j.powtec.2021.02.031 - DOI
LinkOut - more resources
Full Text Sources

