Naringenin attenuates slow-transit constipation by regulating the AMPK/mTOR/ULK1 signalling pathway: in vivo and in vitro studies
- PMID: 40599805
- PMCID: PMC12209368
- DOI: 10.3389/fphar.2025.1550458
Naringenin attenuates slow-transit constipation by regulating the AMPK/mTOR/ULK1 signalling pathway: in vivo and in vitro studies
Abstract
Background: Slow-transit constipation (STC) is a widespread functional gastrointestinal condition distinguished by decreased colonic motility as an essential clinical characteristic. The excessive autophagy of interstitial cells of Cajal (ICCs) causes phenotypic changes and functional abnormalities, which are important in colonic dysmotility. Naringenin (NAR) has been shown to regulate gastrointestinal motility disorders. The present study aimed to elucidate the regulatory role of naringenin in autophagy in STC and its underlying mechanism.
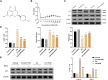
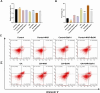
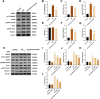
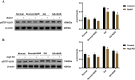
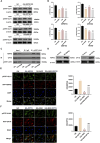
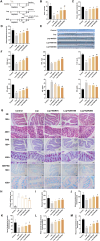
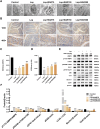
Methods: In vitro, ICCs were stimulated with L-glutamic acid (GA) to induce autophagy and treated with NAR. A CCK8 assay was performed to evaluate the cytotoxic effect of NAR. Annexin V-FITC/PI staining was used to examine NAR apoptosis. The expression of the autophagy markers Beclin1 and LC3B, as well as proteins related to the AMPK/mTOR/ULK1 pathway was investigated through quantitative PCR, Western blot analysis and immunofluorescence staining. The small interfering RNA (siRNA) technique was used to knockdown selective autophagy receptors (NDP52, OPTN, NBR1, and p62) in ICCs. Coimmunoprecipitation (co-IP) was used to evaluate the binding of pS757-ULK1 to the autophagy receptors NDP52 and OPTN in ICCs. Immunofluorescence (IF) staining was performed to observe the colocalization of pS757-ULK1 with exogenous NDP52 and OPTN in ICCs. In vivo, male C57BL/6 mice were administered loperamide (10 mg/kg) to establish a constipation model and then treated with NAR (75/150/300 mg/kg) for 2 weeks. Finally, colonic tissues were collected for a histological analysis and immunohistochemical for cell growth factor receptor kit (c-Kit) and anoctamin-1 (ANO1).
Results: Our results indicated that NAR improved the survival and apoptosis of ICCs after GA by inhibiting autophagy through the partial suppression of the AMPK/mTOR/ULK1 signalling pathway. Moreover, NAR inhibited the autophagic degradation of pS757-ULK1 by weakening the interactions between pS757-ULK1 and the selective autophagy receptor genes NDP52 and OPTN. Further research revealed that NAR could increase the moisture content of faeces; increase the rate of small intestinal propulsion in mice; increase the serum concentrations of excitatory neurotransmitters such as GAS, 5-HT, MTL, and SP; and increase the expression levels of ANO1 and c-Kit in the colon, and the molecular mechanism was consistent with the in vitro results.
Conclusion: NAR attenuates the AMPK/mTOR/ULK1 pathway in ICCs, thereby improving STC colonic dysmotility and underscoring its promise as a therapeutic option for STC.
Keywords: AMPK/mTOR/ULK1 signalling pathway; autophagy; interstitial cells of Cajal; naringenin; slow transit constipation.
Copyright © 2025 Wang, Wang, Qian, Sun, Yang, Su and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Naringenin attenuates slow-transit constipation by regulating the AMPK/mTOR/ULK1 signaling pathway: In vivo and in vitro studies.J Nutr Biochem. 2025 Jun 24:110013. doi: 10.1016/j.jnutbio.2025.110013. Online ahead of print. J Nutr Biochem. 2025. PMID: 40571066
-
miR-210 Regulates Autophagy Through the AMPK/mTOR Signaling Pathway, Reduces Neuronal Cell Death and Inflammatory Responses, and Enhances Functional Recovery Following Cerebral Hemorrhage in Mice.Neurochem Res. 2025 Jun 5;50(3):180. doi: 10.1007/s11064-025-04434-7. Neurochem Res. 2025. PMID: 40471451 Free PMC article.
-
Galactin-8 DNA methylation mediates macrophage autophagy through the MAPK/mTOR pathway to alleviate atherosclerosis.Sci Rep. 2025 Jan 2;15(1):603. doi: 10.1038/s41598-024-85036-1. Sci Rep. 2025. PMID: 39747459 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Research progress on the regulation of interstitial cell of Cajal autophagy and apoptosis crosstalk by traditional Chinese medicine in gastrointestinal motility disorders.J Ethnopharmacol. 2025 Jul 24;351:120128. doi: 10.1016/j.jep.2025.120128. Epub 2025 Jun 11. J Ethnopharmacol. 2025. PMID: 40513924 Review.
References
-
- An H. K., Kim K. S., Lee J. W., Park M. H., Moon H. I., Park S. J., et al. (2014). Mimulone-induced autophagy through p53-mediated AMPK/mTOR pathway increases caspase-mediated apoptotic cell death in A549 human lung cancer cells. PloS one 9 (12), e114607. 10.1371/journal.pone.0114607 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

