Multiple myeloma inhibitory effects of natural compounds: enhancement through nanoparticle carriers
- PMID: 40599807
- PMCID: PMC12209220
- DOI: 10.3389/fphar.2025.1589090
Multiple myeloma inhibitory effects of natural compounds: enhancement through nanoparticle carriers
Abstract
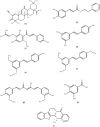
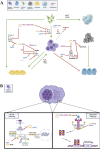
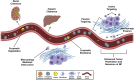
Natural compounds have emerged as promising therapeutic agents for treating cancers such as multiple myeloma (MM). However, poor bioavailability, low stability, and suboptimal targeting often limit their clinical efficacy. Recent advances in nanotechnology have addressed these limitations by utilizing nanoparticle (NP) carriers to enhance the therapeutic potential of natural compounds through improved solubility, stability, and selective delivery to cancer cells. This review explores the inhibitory effects of key natural compounds on MM cells, including 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) and its derivatives, caffeic acid phenethyl ester (CAPE) and its derivatives, xanthohumol (XN) and its derivatives, resveratrol (RSV) and its derivatives, curcumin (CUR), 3,4,5-trihydroxybenzoic acid (gallic acid; GA), and evodiamine (EVO). These compounds exhibit potent anti-proliferative, pro-apoptotic, and anti-inflammatory properties through the modulation of signaling pathways such as NF-κB, STAT3, and PI3K/Akt, which are critical in MM pathogenesis. Despite their therapeutic promise, the clinical application of these natural agents has been hampered by pharmacokinetic challenges. NP carriers, including liposomes, polymeric NPs, and lipid-based nanocarriers, have been engineered to improve these compounds' bioavailability and targeted delivery, enhancing their cytotoxicity against MM cells. For instance, CDDO and its derivatives encapsulated in NPs have demonstrated increased intracellular accumulation and improved inhibition of NF-κB activity. Similarly, NP formulations of CAPE, XN, and RSV have enhanced anti-MM effects through improved stability and sustained drug release. CUR, known for its poor water solubility, has seen its therapeutic potential augmented through NP delivery systems, enabling higher drug concentrations at tumor sites. Though structurally distinct, GA and EVO have benefited from NP-based enhancement, exhibiting improved bioavailability and selective targeting of MM cells. This review highlights the promising role of NP carriers in overcoming the pharmacokinetic limitations of natural compounds, offering new avenues for more effective MM therapies.
Keywords: 2-cyano-3,12-dioxooleana 1,9-dien-28-oic acid (CDDO); caffeic acid phenethyl ester (CAPE); multiple myeloma; nanoparticle carriers; resveratrol (RSV), curcumin (CUR), gallic acid (GA); xanthohumol (XN).
Copyright © 2025 Wong, Staskiewicz, Pruner, Lee, Tucker, Smith, Rogers, Asuni and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Enhancing curcumin's efficacy with zinc oxide nanoparticles: A dynamic duo in combatting disease.Int J Pharm. 2025 Aug 20;681:125889. doi: 10.1016/j.ijpharm.2025.125889. Epub 2025 Jun 23. Int J Pharm. 2025. PMID: 40562286 Review.
-
From Nature to Nanomedicine: Enhancing the Antitumor Efficacy of Rhein, Curcumin, and Resveratrol.Medicina (Kaunas). 2025 May 26;61(6):981. doi: 10.3390/medicina61060981. Medicina (Kaunas). 2025. PMID: 40572668 Free PMC article. Review.
-
Trends in advanced oral drug delivery system for curcumin: A systematic review.J Control Release. 2022 Aug;348:335-345. doi: 10.1016/j.jconrel.2022.05.048. Epub 2022 Jun 9. J Control Release. 2022. PMID: 35654170
-
Synergistically Anti-Multiple Myeloma Effects: Flavonoid, Non-Flavonoid Polyphenols, and Bortezomib.Biomolecules. 2022 Nov 7;12(11):1647. doi: 10.3390/biom12111647. Biomolecules. 2022. PMID: 36358997 Free PMC article.
-
Resveratrol as a Novel Therapeutic Approach for Diabetic Retinopathy: Molecular Mechanisms, Clinical Potential, and Future Challenges.Molecules. 2025 Aug 4;30(15):3262. doi: 10.3390/molecules30153262. Molecules. 2025. PMID: 40807437 Free PMC article. Review.
References
-
- Al-Kinani M. A., Haider A. J., Al-Musawi S. (2021). Design, construction and characterization of intelligence polymer coated core–shell nanocarrier for curcumin drug encapsulation and delivery in lung cancer therapy purposes. J. Inorg. Organomet. Polym. Mater. 31, 70–79. 10.1007/s10904-020-01672-w - DOI
-
- Alsareii S. A., Manaa Alamri A., Alasmari M. Y., Bawahab M. A., Mahnashi M. H., Shaikh I. A., et al. (2022). Synthesis and characterization of silver nanoparticles from Rhizophora apiculata and studies on their wound healing, antioxidant, anti-inflammatory, and cytotoxic activity. Molecules 27, 6306. 10.3390/molecules27196306 - DOI - PMC - PubMed
-
- Altayli E., Koru Ö., Öngörü Ö., İde T., Açikel C., Sarper M., et al. (2015). An in vitro and in vivo investigation of the cytotoxic effects of caffeic acid (3,4-dihydroxycinnamic acid) phenethyl ester and bortezomib in multiple myeloma cells. Turk J. Med. Sci. 45, 38–46. 10.3906/sag-1401-127 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

