Mechanical scratch injury on differentiated motor neuron of NSC-34 cells as an in vitro model for evaluation of neuroregeneration potential of NeuroAiD II (MLC901)
- PMID: 40599870
- PMCID: PMC12209141
- DOI: 10.1007/s44164-024-00070-7
Mechanical scratch injury on differentiated motor neuron of NSC-34 cells as an in vitro model for evaluation of neuroregeneration potential of NeuroAiD II (MLC901)
Abstract
Background: Spinal cord regeneration is considered an ultimate achievement in the field of neuroscience. In vitro, neural stem cell (NSC-34) motor neuron-like cell cultures are powerful tools to study specific molecular pathways involved in neurogenesis.
Purpose: We aimed to demonstrate the usefulness of the in vitro injury model using the mechanical scratch method and to evaluate the effect of MLC901 in injured neuronal cells.
Methods: In this study, retinoic acid (RA) (1 µM and 10 µM) and 30 µM prostaglandin E2 (PGE2) induction was used to facilitate NSC-34 differentiation into motor neurons (MN). The MN was scratched and treated with different concentrations of NeuroAiD II (MLC901). The time-lapse assay, the AKT/P13K pathway analysis, and Immunocytochemistry (ICC) were performed.
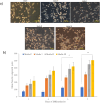
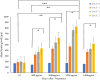
Results: The results showed that NSC-34 cell lines were differentiated into mature MN using RA (7 days) and PGE2 (5 days). The mechanical scratch injury model damaged the MN at the scratch area. The time-lapse assay showed treated cells (T) at conc. In total, 1000 and 1200 µg/mL for MLC 901 showed significantly higher neurite outgrowth as compared to untreated cells (UT). The AKT/PI3K pathway analysis showed higher expression of regenerative markers (p-AKT, p-GSK3β, ATF-3, GAP43, p53, and elF2β) at concentrations of 1200 µg/mL than UT.
Conclusion: The study showed that the in vitro injury model using mechanical scratch is a useful tool to induce neurodegeneration and may be used to evaluate regenerative treatment options.
Supplementary information: The online version contains supplementary material available at 10.1007/s44164-024-00070-7.
Keywords: Mechanical injury; NSC-34 cells; Nerve regeneration; NeuroAiD II (MLC901); Neurodegeneration; PI3K-AKT pathway.
© The Author(s), under exclusive licence to Springer Nature Switzerland AG 2024. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures






Similar articles
-
Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972056 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis.Cochrane Database Syst Rev. 2022 May 20;5(5):CD006981. doi: 10.1002/14651858.CD006981.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593746 Free PMC article.
-
Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD012574. doi: 10.1002/14651858.CD012574.pub2. Cochrane Database Syst Rev. 2022. PMID: 36477774 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- Slovinska L, Blasko J, Nagyova M, Szekiova E, Cizkova D. In vitro models of spinal cord injury. Recovery of motor function following spinal cord injury. IntechOpen; 2016.
-
- Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
