An artificial intelligence-derived metabolic network predicts psychosis in Alzheimer's disease
- PMID: 40599906
- PMCID: PMC12209852
- DOI: 10.1093/braincomms/fcaf159
An artificial intelligence-derived metabolic network predicts psychosis in Alzheimer's disease
Abstract
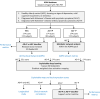
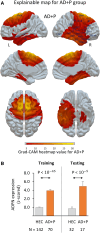
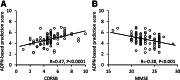
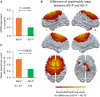
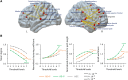
The delusions and hallucinations that characterize Alzheimer's disease psychosis (AD + P) are associated with violence towards caregivers and an accelerated cognitive and functional decline whose management relies on the utilization of medications developed for young people with schizophrenia. The development of novel therapies requires biomarkers that distinguish AD + P from non-psychotic Alzheimer's disease. We investigated whether there might exist a brain metabolic network that distinguishes AD + P from non-psychotic Alzheimer's disease that could be used as a biomarker to predict and track the course of AD + P for use in clinical trials. Utilizing F-18 fluorodeoxyglucose positron emission tomography scans from cohorts of cognitively healthy elderly (N = 174), those with Alzheimer's disease without psychosis (N = 174) and those with AD + P (N = 88) participating in the Alzheimer's Disease Neuroimaging Initiative study, we employed a convolutional neural network to identify and validate the Alzheimer's Psychosis Network. We analysed network progression, clinical correlations and psychosis prediction using expression scores and network organization using graph theory. The Alzheimer's Psychosis Network accurately distinguishes AD + P from controls (97%), with increasing scores correlating with cognitive decline. The Alzheimer's Psychosis Network-based approach predicts psychosis in Alzheimer's disease with 77% accuracy and identifies specific brain regions and connections associated with psychosis. Alzheimer's Psychosis Network expression was found to be associated with increased cognitive and functional decline that characterizes AD + P. The increased metabolic connectivity between motor and language/social cognition regions in AD + P may drive delusions and agitated behaviour. Alzheimer's Psychosis Network holds promise as a biomarker for AD + P, aiding in treatment development and patient stratification.
Keywords: Alzheimer’s disease psychosis; biomarker; convolutional neural network; explainable AI; metabolic brain networks.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Stern Y, Mayeux R, Sano M, Hauser WA, Bush T. Predictors of disease course in patients with probable Alzheimer’s disease. Neurology. 1987;37(10):1649–1653. - PubMed
-
- Stern Y, Albert M, Brandt J, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer’s disease. Neurology. 1994;44(12):2300–2300. - PubMed
LinkOut - more resources
Full Text Sources
