Immune cells in Alzheimer's disease: insights into pathogenesis and potential therapeutic targets
- PMID: 40600183
- PMCID: PMC12207208
- DOI: 10.1515/mr-2024-0064
Immune cells in Alzheimer's disease: insights into pathogenesis and potential therapeutic targets
Abstract
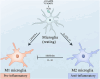
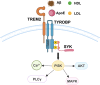
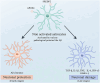
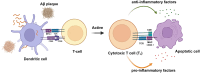
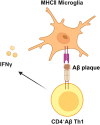
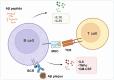
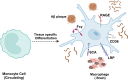
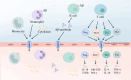
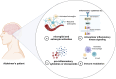
Alzheimer's disease (AD) is a chronic neurodegenerative disorder for which there are currently no effective treatment options. Increasing evidence suggests that AD is a systemic disease closely associated with the immune system, not merely a central nervous system (CNS) disorder. Immune cells play crucial roles in the onset and progression of AD. Microglia and astrocytes are the primary inflammatory cells in the brain that can sensitively detect changes in the internal environment and transform into different phenotypes to exert differing effects at various stages of AD. Peripheral immune cells, such as T cells, B cells, monocytes/macrophages, and neutrophils can also be recruited to the CNS to mediate the inflammatory response in AD. As such, investigating the role of immune cells in AD is particularly important for elucidating its specific pathogenesis. This review primarily discusses the roles of central innate immune cells, peripheral immune cells, and the interactions between central and peripheral immune cells in the development of neuroinflammation in AD. Furthermore, we listed clinical trials targeting AD-associated neuroinflammation, which may represent a promising direction for developing effective treatments for AD in the future.
Keywords: Alzheimer’s disease; immune cell; inflammation; therapeutics.
© 2024 the author(s), published by De Gruyter, Berlin/Boston.
Conflict of interest statement
Conflict of interest: Authors state no conflict of interest.
Figures

References
-
- Huimin C, Xiaofeng F, Shuiyue Q, Ziye R, Changbiao C, Longfei J. Amyloid-β-targeted therapies for Alzheimer’s disease: currently and in the future. Ageing Neurodegener Dis. 2023;3:13. doi: 10.20517/and.2023.16. - DOI
-
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
