Cheese starter cultures attenuate inflammation in the in vitro Caco-2 model
- PMID: 40600210
- PMCID: PMC12207260
- DOI: 10.3934/microbiol.2025017
Cheese starter cultures attenuate inflammation in the in vitro Caco-2 model
Abstract
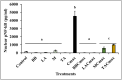
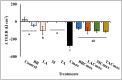
Chronic inflammation is identified to be an underlying pathophysiology in different conditions including inflammatory bowel disease (IBD). Since the aberrant interaction of the mucosal immune system with the dysbiotic flora has been reported to contribute to IBD development, probiotics have been studied for potential prophylaxis and treatment. In this regard, fermented dairy foods are a rich source of probiotics and bioactive compounds. However, limited studies have determined the impact of fermented dairy products in the context of chronic inflammation. In particular, a potential role for dairy starter cultures is not well studied. Hence, in this study we evaluated the anti-inflammatory effect of two cheese starter cultures (Lactococcus lactis subsp. lactis M58 and Streptococcus thermophilus TA 61) in comparison with commercial probiotic strains (Bifidobacterium animalis subsp. lactis BB-12, Lactobacillus acidophilus LA-5) using the Cmax-induced Caco-2 inflammation model. Specifically, we characterized their ability to attenuate inflammatory response via modulation of IL-8 secretion, NF-κB activation, barrier integrity (TEER), and tight junction gene expression. Overall, pre-exposure to the starter cultures before Cmax treatment significantly reduced the activation and nuclear translocation of NF-κB, compared to cytokine control (P < 0.05). Further, the reduction in pNF-κB was found to be associated with a significant reduction in IL 8 secretion (P < 0.05). Moreover, the cultures protected the Caco-2 monolayer from inflammation-induced increase in permeability by upregulating the genes associated with ZO-1 and occludin production. Furthermore, the protective effect of the starter cultures was comparable to that of the commercial probiotics with known anti-inflammatory properties. Therefore, cheese starter cultures could be a potential strategy against chronic gut inflammation.
Keywords: Caco-2; anti-inflammatory potential; cheese starter cultures; in vitro; inflammation; inflammatory bowel disease; intestinal epithelial cells.
© 2025 the Author(s), licensee AIMS Press.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
