Lymphatic dysfunction correlates with inflammation in a mouse model of amyotrophic lateral sclerosis
- PMID: 40600271
- PMCID: PMC12309905
- DOI: 10.1242/dmm.052148
Lymphatic dysfunction correlates with inflammation in a mouse model of amyotrophic lateral sclerosis
Abstract
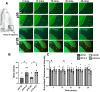
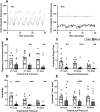
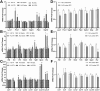
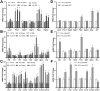
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive, ultimately fatal neurodegenerative disease, without effective modifying treatments. It affects both lower and upper motor neurons, causing skeletal muscle denervation and paralysis. Regardless of the mechanisms that initiate and drive ALS, chronic neuroinflammation and systemic immune system activation play key roles in disease progression. The lymphatic system is a network of vessels and organs essential for immune surveillance, tissue fluid balance and lipid absorption, critical for the resolution and progression of inflammation in the periphery. Its recent rediscovery in the central nervous system raises the possibility of it playing similar roles in neurological and neurodegenerative diseases featuring prominent neuroinflammation, such as ALS. We hypothesized that the structure and function of lymphatics are compromised in the most widely used murine model of ALS, the SOD1-G93A mouse. We found that these mice exhibit lymph transport dysfunction, diminished intrinsic lymphatic vessel tonic and phasic contractions, and an association between inflammation and lymphatic marker upregulation, despite absence of major structural changes in lymphatic network coverage in key affected tissues in the disease, skeletal muscle and spinal cord.
Keywords: Amyotrophic lateral sclerosis; Lymphangiogenesis; Lymphatics; Neuroinflammation; Systemic inflammation.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

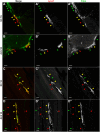
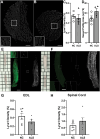
References
-
- Antila, S., Chilov, D., Nurmi, H., Li, Z., Näsi, A., Gotkiewicz, M., Sitnikova, V., Jäntti, H., Acosta, N., Koivisto, H.et al. (2024). Sustained meningeal lymphatic vessel atrophy or expansion does not alter Alzheimer's disease-related amyloid pathology. Nat. Cardiovasc. Res. 3, 474-491. 10.1038/s44161-024-00445-9 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

