Response to First-Line Chemotherapy Predicts Response to Maintenance Avelumab Therapy in Japanese Patients With Advanced Urothelial Carcinoma
- PMID: 40600395
- PMCID: PMC12503202
- DOI: 10.1111/iju.70162
Response to First-Line Chemotherapy Predicts Response to Maintenance Avelumab Therapy in Japanese Patients With Advanced Urothelial Carcinoma
Abstract
Objectives: The association between the response to first-line chemotherapy and maintenance of avelumab remains unclear. We identified factors associated with the response to avelumab in patients with advanced urothelial carcinoma using real-world data.
Methods: We retrospectively enrolled 100 patients with advanced urothelial carcinoma treated with maintenance avelumab therapy between March 2021 and April 2024 at Nagoya University and nine affiliated hospitals. The complete/partial-response group was defined as patients with complete response or partial response as the best response to first-line chemotherapy. The stable disease group was defined as patients with stable disease as the best response to first-line chemotherapy.
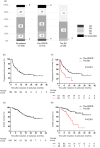
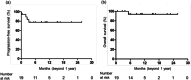
Results: Seven patients (7.0%) achieved complete response, 65 (65.0%) achieved partial response, and 28 (28.0%) achieved stable disease as the best response to first-line chemotherapy. Regarding avelumab therapy, the complete/partial-response group had significantly better progression-free survival than the stable disease group (median: 11.1 vs. 3.2 months, p < 0.001). In multivariate analyses, the best response to first-line chemotherapy was the only independent risk factor for progression-free survival (hazard ratio = 1.844, 95% confidence interval = 1.002-3.394; p = 0.049). Overall survival was significantly shorter in the stable disease group than in the complete/partial-response group (median: 14.1 months vs. not reached, p < 0.001). Multivariate analyses revealed significant associations between poor overall survival and performance status (hazard ratio = 2.175, 95% confidence interval = 1.030-4.592; p = 0.042) and the best response to first-line chemotherapy (hazard ratio = 4.174, 95% confidence interval = 1.975-8.824; p < 0.001).
Conclusions: The best response to first-line chemotherapy may predict the clinical outcome of patients with advanced urothelial carcinoma treated with avelumab.
Keywords: avelumab; carcinoma; immunotherapy; prognosis; risk factors.
© 2025 The Author(s). International Journal of Urology published by John Wiley & Sons Australia, Ltd on behalf of The Japanese Urological Association.
Conflict of interest statement
Shusuke Akamatsu is an Editorial Board member of the International Journal of Urology and a co‐author of this article. To minimize bias, he was excluded from all editorial decision‐making related to the acceptance of this article for publication. The other authors declare no conflicts of interest.
Figures
References
-
- von der Maase H., Sengelov L., Roberts J. T., et al., “Long‐Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer,” Journal of Clinical Oncology 23 (2005): 4602–4608. - PubMed
-
- Powles T., Park S. H., Voog E., et al., “Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma,” New England Journal of Medicine 383 (2020): 1218–1230. - PubMed
-
- Powles T., Valderrama B. P., Gupta S., et al., “Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer,” New England Journal of Medicine 390 (2024): 875–888. - PubMed
-
- Bellmunt J., Choueiri T. K., Fougeray R., et al., “Prognostic Factors in Patients With Advanced Transitional Cell Carcinoma of the Urothelial Tract Experiencing Treatment Failure With Platinum‐Containing Regimens,” Journal of Clinical Oncology 28 (2010): 1850–1855. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

