Quality Assurance in Cervical Cancer Screening: Evaluation of Sample Adequacy in HPV DNA Testing
- PMID: 40600419
- PMCID: PMC12216795
- DOI: 10.1002/jmv.70482
Quality Assurance in Cervical Cancer Screening: Evaluation of Sample Adequacy in HPV DNA Testing
Abstract
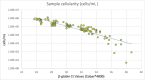
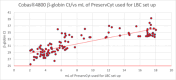
In HPV-primary screening, sample quality significantly influences test accuracy. Unlike cytology-based screening, no consensus guidelines presently exist for sample quality assessment in HPV testing. This study aims to evaluate the impact of sample cellularity on HPV testing. A total of 37 592 liquid-based cytology (LBC) samples from women undergoing HPV-primary screening (aged 30-64, median 48; IQR: 40-56 years) were analyzed using Cobas 4800 HPV Test (Roche). Sample adequacy was assessed by the assay's β-globin internal control and by an independent quantitative cellularity assessment (OncoPredict HPV, Hiantis). HPV positivity rates (PR) were stratified according to β-globin Ct values. Among the analyzed samples, 50.0%, 47.1%, 2.3%, and 0.6% had β-globin Ct values of ≤ 28, > 28 to ≤ 32, > 32 to ≤ 34, and > 34, respectively. Overall HPV-PR was 7.7% (2891/37 592). PR reached 9.7% in samples with β-globin ≤ 28 Ct (1820/18 801), decreasing markedly to 1.4% for β-globin > 34 Ct (3/214), (p < 0.001). Quantitative analysis showed that Cobas 4800 β-globin Ct = 34 corresponds to approximately 1.5 × 10^3 nucleated cells/reaction. A subset of 195 HPV-negative samples with β-globin Ct ≥ 34 was evaluated by liquid based cytology (LBC): 19% had inadequate cellularity according to LBC guidelines, 8% were ≥ ASC-US and 73% NILMs. 65% of adequate LBC showed cellular atrophy. These findings emphasize the importance of assessing cellularity in HPV-screening to avoid potentially false-negative results due to inadequate samples. Future research should focus on establishing standardized cellularity thresholds to improve screening accuracy.
Keywords: HPV‐DNA test quality assurance (QA); cervical cancer screening; sample adequacy; sample cellularity.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
C. E. C. institution has received research grants and/or gratis consumables from Beckton Dickinson, Copan Italia, Seegene, Novosanis and Fujirebio. C. E. C. has received speaker honoraria and/or travel funds from Seegene, Beckton Dickinson, Copan Italia. C. E. C. is a minority shareholder of Hiantis Srl.
Figures
Similar articles
-
[Health technology assessment report. Use of liquid-based cytology for cervical cancer precursors screening].Epidemiol Prev. 2012 Sep-Oct;36(5 Suppl 2):e1-e33. Epidemiol Prev. 2012. PMID: 23139163 Italian.
-
The Utility of an Human Papillomavirus Genotype Assay for Cancer Screening in Self-Collected Urine and Vaginal Samples from Japanese Women.Gynecol Obstet Invest. 2025;90(2):143-152. doi: 10.1159/000541641. Epub 2024 Oct 7. Gynecol Obstet Invest. 2025. PMID: 39374596 Free PMC article.
-
Cytology versus HPV testing for cervical cancer screening in the general population.Cochrane Database Syst Rev. 2017 Aug 10;8(8):CD008587. doi: 10.1002/14651858.CD008587.pub2. Cochrane Database Syst Rev. 2017. PMID: 28796882 Free PMC article.
-
Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force.Ann Intern Med. 2011 Nov 15;155(10):687-97, W214-5. doi: 10.7326/0003-4819-155-10-201111150-00376. Epub 2011 Oct 17. Ann Intern Med. 2011. PMID: 22006930
-
Exponential uptake of HPV self-collected cervical screening testing 2 years since universal availability in Victoria, Australia.BMC Med. 2025 Jul 1;23(1):389. doi: 10.1186/s12916-025-04219-3. BMC Med. 2025. PMID: 40598474 Free PMC article.
References
-
- Ronco G., Biggeri A., Confortini M., et al., “Health Technology Assessment Report: HPV DNA‐Based Primary Screening for Cervical Cancer Precursors,” supplement, Epidemiologia E Prevenzione 36, no. 3–4 S1 (2012): e1–e72. - PubMed
-
- Ronco G., Dillner J., Elfström K. M., et al., “Efficacy of HPV‐Based Screening for Prevention of Invasive Cervical Cancer: Follow‐Up of Four European Randomised Controlled Trials,” Lancet 383 (2014): 524–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous