TransplantLines, a biobank and cohort study of solid organ transplant recipients and donors
- PMID: 40601244
- PMCID: PMC12374880
- DOI: 10.1007/s10654-025-01258-1
TransplantLines, a biobank and cohort study of solid organ transplant recipients and donors
Abstract
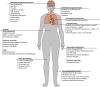
The TransplantLines Biobank and Cohort Study (NCT03272841) is an ongoing prospective study conducted at the University Medical Centre Groningen, The Netherlands. TransplantLines aims to identify risk factors and biomarkers associated with health problems following solid organ transplantation and donation. Additionally, the study seeks to develop new interventions to reduce symptom burden and improve long-term outcomes, including health-related quality of life, cardiovascular complications, graft failure, and mortality. It includes recipients of (combined) heart, liver, lung, kidney, pancreas, and small bowel transplants, as well as living liver and kidney donors, and deceased (multi-)organ donors. The biobank contains a wide range of biomaterials including whole blood, serum, EDTA-plasma, buffy coat, 24-h urine samples, faeces, hair, nails, and tissues. Data collection includes physical and cognitive assessments, extensive laboratory analysis, metagenomic sequencing, and questionnaires. TransplantLines, initiated in 2015, consists of 5143 participants as of October 2024, among 2312 (45%) females. The mean age was 50 (± 16) years at transplantation, 55 (± 11) years at living donation and 56 (± 15) years at deceased donation. Both cross-sectional and longitudinal biomaterials and data are included. For recipients, longitudinal biomaterials and data were collected at: pre-transplantation, at transplantation, and at 3, 6, 12, 24, and 60 months post-transplantation. For living donors, data were collected at pre-donation, donation, 3 months post-donation, and/or 5 or 10 years post-donation.
Keywords: Biobank; Cohort profile; Solid organ donation; Solid organ transplantation; TransplantLines.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: MB reports a grant from CSL Vifor, outside the submitted work. Additionally, MB reports consulting fees paid to his institution by Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, CSL Vifor, Kyowa Kirin Pharma, Lilly and Sanofi Genzyme. KD reports consulting fees from Abbot, Astra Zeneca, Boehringer Ingelheim, Novartis, Echosense, FIRE1 and Reprieve paid to his institution. TG reports participation on a data safety monitoring board or advisory board from Chiesi pharmaceuticals, payments paid to his institution. VM reports a VENI research grant by the Dutch Research Council (NWO; grant #09150161810030), a Research grant from the Dutch Ministry of Economic Affairs (Health ~ Holland Public Private Partnership grant #PPP31 2019–024), and a Research grant from the Dutch Society for Gastroenterology (NVGE #01–2021), all outside the submitted work. VM further reports speakers fees paid to his institution by Astellas, Chiesi, and XVIVO. All above reported conflict of interest are not directly involved in this manuscript. The remaining authors do not have conflicts of interests to report. Ethics approval: The study protocol has been approved by the local Institutional Review Board (METc 2014/077), adheres to the UMCG Biobank Regulation, and complies with the WMA Declaration of Helsinki and the Declaration of Istanbul [6]. Consent to participate: Written informed consent was obtained from all participants prior to inclusion for all recipients and living donors. For deceased donors, informed consent was obtained from their relatives, following the applicable regulations and agreements of the Dutch Transplant Foundation (NTS) regarding participation and use in scientific research. TransplantLines Investigators: Coby Annema, Stefan P Berger, Hans Blokzijl, Frank AJA Bodewes, Marieke T de Boer, Kevin Damman, Martin H de Borst, Arjan Diepstra, Gerard Dijkstra, Rianne M Douwes, Caecilia SE Doorenbos, Michele F Eisenga, Michiel E Erasmus, C Tji Gan, Antonio W Gomes-Neto, Eelko Hak, Bouke G Hepkema, Marius C van den Heuvel, Jip Jonker, Frank Klont, Tim J Knobbe, Daan Kremer, Coretta van Leer-Buter, Henri GD Leuvenink, Marco van Londen, Willem S Lexmond, Vincent E de Meijer, Hubert GM Niesters, Gertrude J Nieuwenhuis-Moeke, L Joost van Pelt, Robert A Pol, Anna M Posthumus, Adelita V Ranchor, Jan Stephan F Sanders, Marion J Siebelink, Riemer JHJA Slart, J Cas Swarte, Daan J Touw, Charlotte A te Velde-Keyzer, Erik AM Verschuuren, Michel J Vos, Rinse K Weersma, Stephan JL Bakker.
Figures


References
-
- Carbone M, M. Neuberger J. Solid Organ Transplantation. Regenerative Medicine Applications in Organ Transplantation, Elsevier; 2014, 10.1016/B978-0-12-398523-1.00002-1.
-
- Knobbe TJ, Kremer D, Douwes RM, et al. Proton pump inhibitor use, fatigue, and health-related quality of life in kidney transplant recipients: results from the transplantlines biobank and cohort study. Am J Kidney Dis. 2023;82:189-201.e1. 10.1053/j.ajkd.2022.12.012. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

