Hurdles for the Delivery of Multinational Randomized Clinical Trials
- PMID: 40601320
- PMCID: PMC12223866
- DOI: 10.1001/jamanetworkopen.2025.18503
Hurdles for the Delivery of Multinational Randomized Clinical Trials
Abstract
Importance: Ethical, administrative, regulatory, and logistical (EARL) procedures can hamper clinical trial delivery. Quantification of these hurdles is rare, prohibiting identification of areas for improvement.
Objective: To identify and quantify EARL hurdles in trial delivery before and during the COVID-19 pandemic.
Design, setting, and participants: This cohort study used data from the ongoing Randomized Embedded Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia to enable comparison of EARL procedures for multiple protocols across 19 European countries in the pre-COVID-19 pandemic (February 19, 2016 to March 10, 2020) and COVID-19 pandemic (March 11, 2020, to May 4, 2023) periods. Data were analyzed from November 2024 to March 2025 with contracts and protocol submissions as the units of analysis.
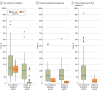
Main outcome and measures: Time to (1) site contract completion, (2) regulatory and ethical approval (TTA), and (3) first patient in (FPI). The UK was compared with non-UK countries because of its distinct research infrastructure.
Results: There were 257 fully signed first contracts with study sites for analysis. In the UK, contract completion times decreased by 97% (95% CI, 95% to 98%), from a median (IQR) of 196 (154 to 250) days in the pre-COVID-19 pandemic period to 5 (1 to 11) days during the COVID-19 pandemic. In non-UK countries, median (IQR) contract completion times were 224 (119 to 412) days and 183 (62 to 291) days before and during the COVID-19 pandemic, respectively (relative difference, -18%; 95% CI, -43% to 52%). In total, 44 interventions in 16 domains were submitted, yielding 232 protocol approvals for analysis. During the COVID-19 pandemic, median (IQR) TTA was 8 (5 to 31) days in the UK and 115 (47 to 103) days in non-UK countries (median difference, 107 days; 95% CI, 76 to 123 days), with large variation across non-UK countries. Time between approval and FPI during the COVID-19 pandemic was, on average, 3 months faster in the UK compared with non-UK countries (median difference, 90 days; 95% CI, 42 to 141 days).
Conclusions and relevance: This study found that EARL procedures were lengthy and variable between countries, reflecting different interpretations of trial regulations, with faster processes in the UK. These findings underscore the need to streamline processes across European countries to improve trial efficiency, in particular during future public health emergencies such as pandemics.
Conflict of interest statement
Figures

Comment in
References
-
- National Health Service . Novel coronavirus: clinical trials. Published April 1, 2020. Accessed 6 December, 2024. https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID...
-
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. doi: 10.1136/bmj.39335.541782.AD - DOI - PMC - PubMed
-
- World Health Organization . Statement on the fifteenth meeting of the IHR (2005) emergency committee regarding the COVID-19 pandemic. Published May 5, 2023. Accessed May 28, 2025. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meet...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

